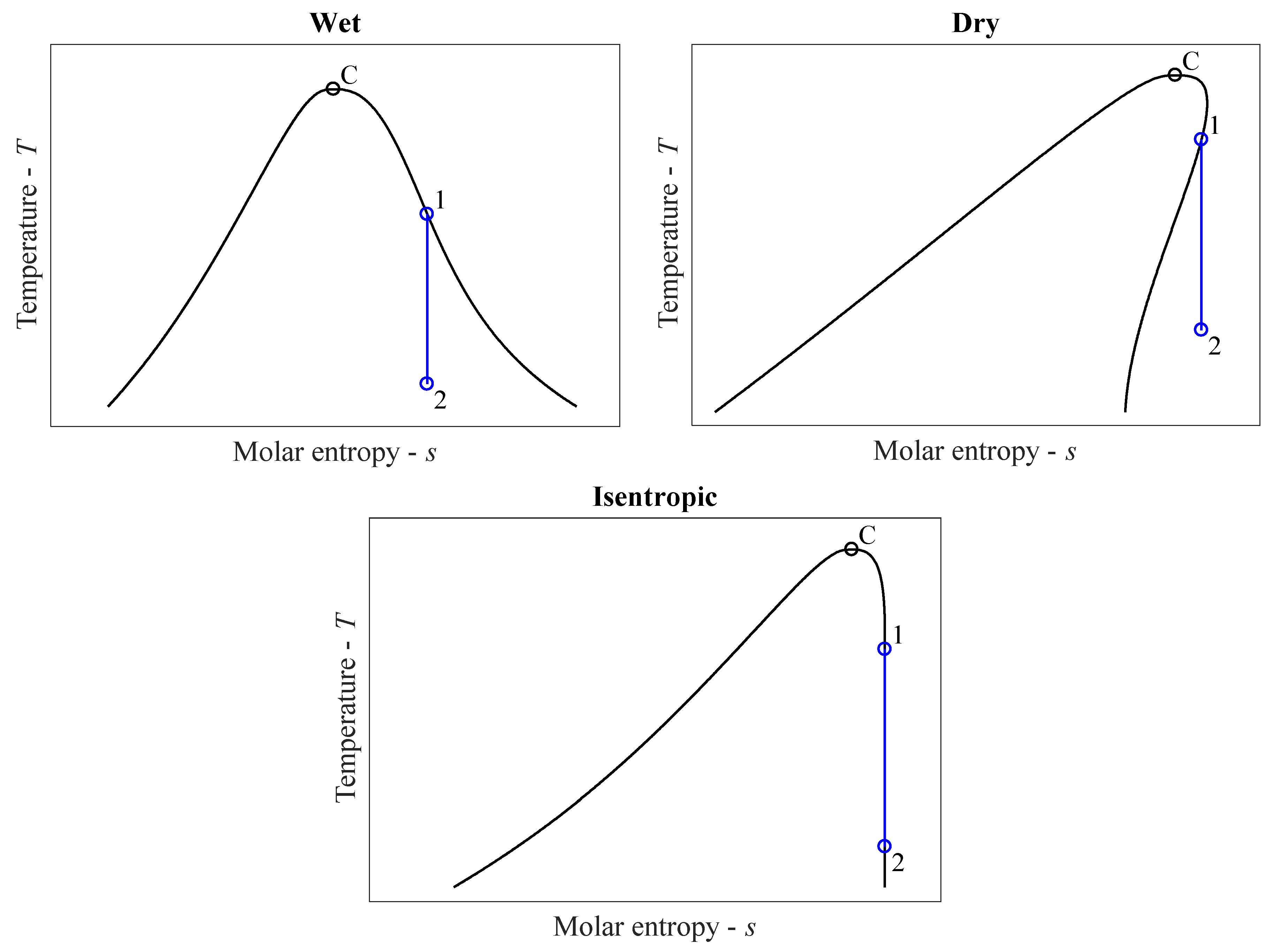
34 in the diagram which letter represents the transition from gas to liquid
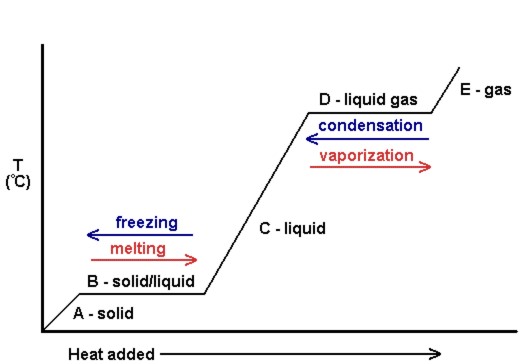
The diagram shows changes of state between solid, liquid, and gas. The atoms of a substance lose energy during a change of state. After the change, the atoms are close together but are able to slide past one another. gas L ON O ОР M z o Р solid liquid Q. THIS IS THE BEST ANSWER 👇. C. Letter O. Explanation: In the diagram, which letter represents the transition from liquid to gas? Segment D. ... In the diagram, which letter represents the transition from solid to liquid? Segment B. THIS SET IS OFTEN IN FOLDERS WITH... Chemistry Unit 5 Practice Test. 20 terms. meaghan__thompson.
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ...

In the diagram which letter represents the transition from gas to liquid
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. PHASE DIAGRAM WORKSHEET Part A - Generic Phase Diagram. Answer the questions below in relation to the following generic phase diagram. 1. Which section represents the solid phase? _____ 2. What section represents the liquid phase? _____ 3. What section represents the gas phase? _____ 4. What letter represents the triple point? Problem Details. In the phase diagram, which transition represents the condensation of a gas into a liquid? (A) B to A. (B) D to B. (C) C to D. (D) A to D. Learn this topic by watching Phase Diagrams Concept Videos.
In the diagram which letter represents the transition from gas to liquid. In the diagram, which letter represents the transition from liquid to gas? (can't show diagram... Sorry :<) D. ... In the diagram, which letter represents the heating of a solid? (same diagram as #17 & #21) A. The reaction in the diagram takes place in an ice calorimeter at 0°C. 6) gas b. plasma liquid c. d. solid 5. Most matter condense a. b. contracts expands c. . solidifies when heated. 6. Matter in which the atoms are held tightly in place is a. gas b. plasma c. liquid @solid 13. Condensation is the phase change in which a substance changes from a. solid to a gas b. solid to a liquid c. liquid to a gas @gas to a ... Phase diagrams critical point where a substance can exist as a liquid and gas. Where the liquid and gas meet. triple point where substance can exist in all three phases of equilibrium. fusion curve melting or freezing represents the transition between solid and liquid vaporization curve condensation represents the transition between gas and liquid 23.01.2022 · Additionally, the peaks of the liquid crystal shrunk until they were no longer present. For example, only peaks of the regular ITR crystal were observed after 87 days. The recrystallized ITR was again the Form I polymorph of ITR. No polymorph other than the Form I, nor the liquid crystal, was ever detected for ITR within this work [31,32,33].
The diagram is divided into three areas, which represent the solid, liquid, and gaseous states of the substance. The best way to remember which area corresponds to each of these states is to remember the conditions of temperature and pressure that are most likely to be associated with a solid, a liquid, and a gas. Question 6. SURVEY. 30 seconds. Q. Letter "C" represents which state of matter on the phase change diagram shown. answer choices. Solid. Liquid. PHASE DIAGRAM WORKSHEET Part A - Generic Phase Diagram. Answer the questions below in relation to the following generic phase diagram. 1. Which section represents the solid phase? _____ 2. What section represents the liquid phase? _____ 3. What section represents the gas phase? _____ 4. What letter represents the triple point? Aug 17, 2021 · Which letter in the diagram represents the transition from liquid to gas? Temperature vs. Amount of Heat Added to a Substance 200 O A m OB D 9 100 C Temperature (°C) C B OD 0 А-100 Amount of heat added
What section represents the gas phase. Phase Diagram Worksheet Name. What section represents the liquid phase. 4 the melting point curve leans slightly to the right has a positive slope indicating that as pressure is increase the melting point of bromine increases. 1 label the following on the phase diagram above. Consider the phase diagram below: 1. Which section represents the solid phase? A 2. Which section represents the liquid phase? C 3. Which section represents the gas phase? B 4. What letter represents the triple point? d 5. What is the temperature and pressure of the triple point? Temperature: 45oC Pressure: 0.5atm 6. What is the normal melting ... Mar 18, 2018 · IF I'm right the answer is D. My diagram shows. A at -50 °C. B at 0 °C. C at 50 °C. D at 100 °C (gas to liquid or liquid to gas) And E at 150 °C. So I hope I'm right because I'm answering the same question. soobee72pl and 47 more users found this answer helpful. Liquid, only Gas, only Phase change For section QR of the graph, state what is happening ... What letter represents the potential energy of the products? What letter represents the heat of reaction (AH)? ... solid, liquid, gas, solid/liquid mix, liquid/gas mix 3. Label the Melting Point and Boilin Point of water (in oc) on the y-axis.
For each of the following questions (16-28), refer to the phase diagram for tastegudum. See Miss Scott for answer key with labels. Label the regions of the diagram that correspond to the solid, liquid, and vapor phases. (Write the names of these phases in the appropriate regions directly on the diagram.)
The diagram below shows particles in a gas, a solid and a liquid. Each arrow, A, B, C and D, represents a change of state. Choose from the following words to complete the sentences below: melting boiling dissolving evaporating softening solidifying condensing separating (a) Change of state A is called _____
Which letter represents a Gas. E. Which letter represents a Solid. A. ... Start studying Phase Change Diagram. Learn vocabulary, terms, and more with flashcards, games, and other study tools. ... Which letter represents a liquid. c. Which line shows condensation. D. Which line represents melting. b.
Vaporization - a phase change from liquid to gas. Vaporization (or condensation) curve - the curve on a phase diagram which represents the transition between the gaseous and liquid states. VOCABULARY LIST D irections: Read each item carefully. Choose the letter of the best answer . 1.
This represents a two-out-of-three-good condition, and so we would expect the PLC to turn on the “Burner lit” indicator light as programmed. From the perspective of the PLC’s rack, we would see the indicator LEDs for sensors B and C lit up on the discrete input card, as well as the indicator LED for the lamp’s output channel: Note 1 : The most likely reason why one out of two …
The line between regions B and C represents the transition. The line between regions A and C represents the transition. The corresponds to the temperature and pressure at which all three phases are in equilibrium with one another. The critical point occurs at the critical, and marks the end of the line that separates the gas and liquid phases.
Academia.edu is a platform for academics to share research papers.
Part A On the phase diagram, which section of curve represents the pressure and temperature values at which ammonia will boil? Express your answer as two letters that lie on a section of the appropriate curve. Hint 1. Describe boiling in terms of phase changes Boiling is the transition from one phase to another with both phases existing together. The phases and direction of change involved in ...
Use the following diagram to answer questions 15-25. Is heat being added or removed from this substance? _____ Which segment represents the point during which the gas is turning into a liquid (g → l)? _____ Which segment represents the point during which the liquid is turning into a solid (l → s)?
Draw lines of equlhbflum between solid and gas, liquid and gas, solid and liquld phases using the numbers provided Label your axes Including units and add all the numbers from the data table I $5 55K 68K 183 K 218K 0.10 atm 50 atm Part 8: Use the following phase diagram or Tastegudum (a compound from another universe) to
Recombinant nanoworms are promising candidates for materials and biomedical applications ranging from the templated synthesis of nanomaterials to multivalent display of bioactive peptides and targeted delivery of theranostic agents. However, molecular design principles to synthesize these assemblies (which are thermodynamically favorable only in a narrow region of the phase …
green section represents the transition between solid and liqu…. blue section represents the transition between gas and liquid. what are the approximate conditions for…. 8 p (bar) and 220K. triple point. where substance can exist in all three phases of equilibrium. 12 Terms. TalbertChem. Phase diagram.
Problem Details. In the phase diagram, which transition represents the condensation of a gas into a liquid? (A) B to A. (B) D to B. (C) C to D. (D) A to D. Learn this topic by watching Phase Diagrams Concept Videos.
PHASE DIAGRAM WORKSHEET Part A - Generic Phase Diagram. Answer the questions below in relation to the following generic phase diagram. 1. Which section represents the solid phase? _____ 2. What section represents the liquid phase? _____ 3. What section represents the gas phase? _____ 4. What letter represents the triple point?
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance.






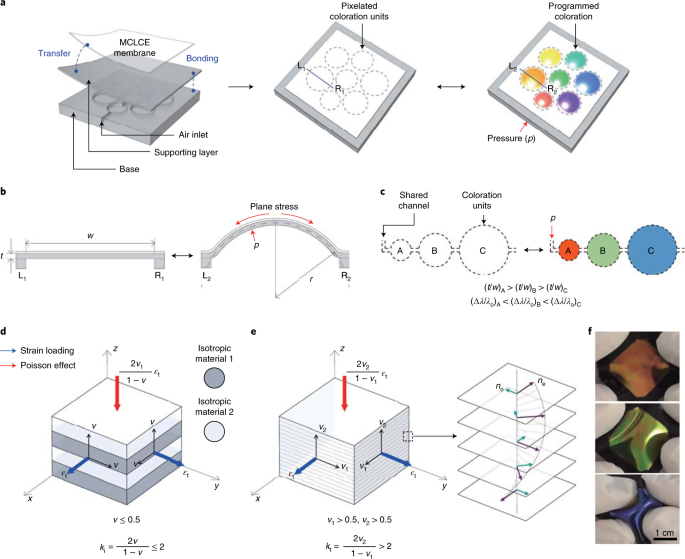
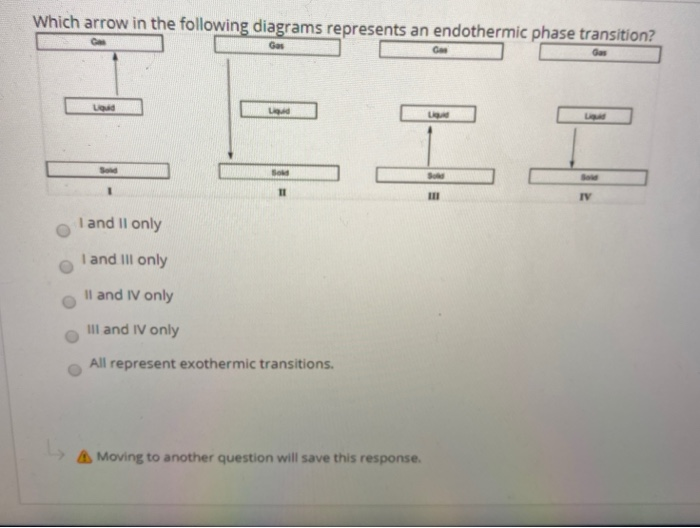

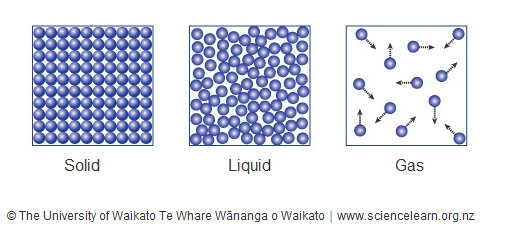




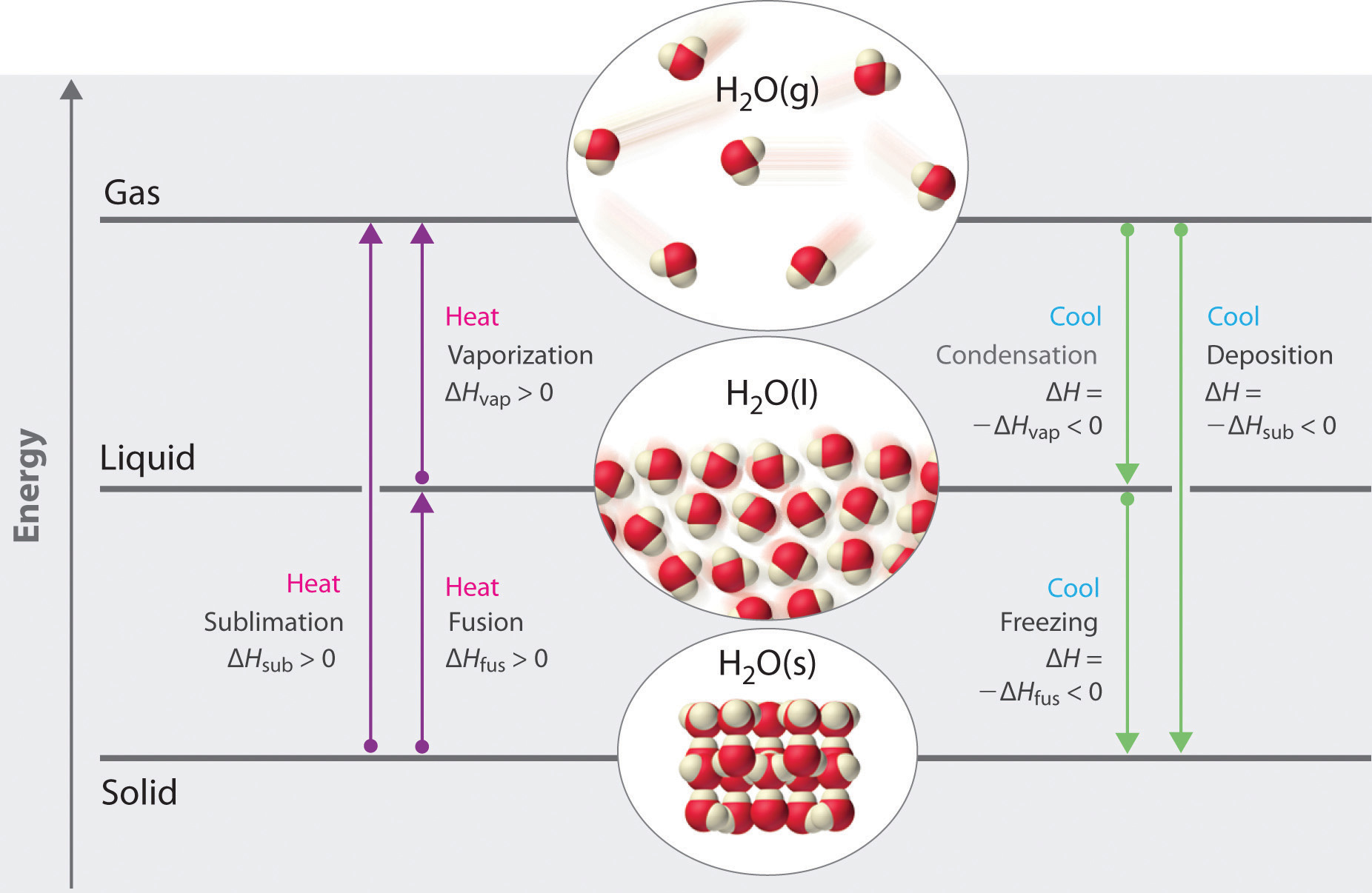


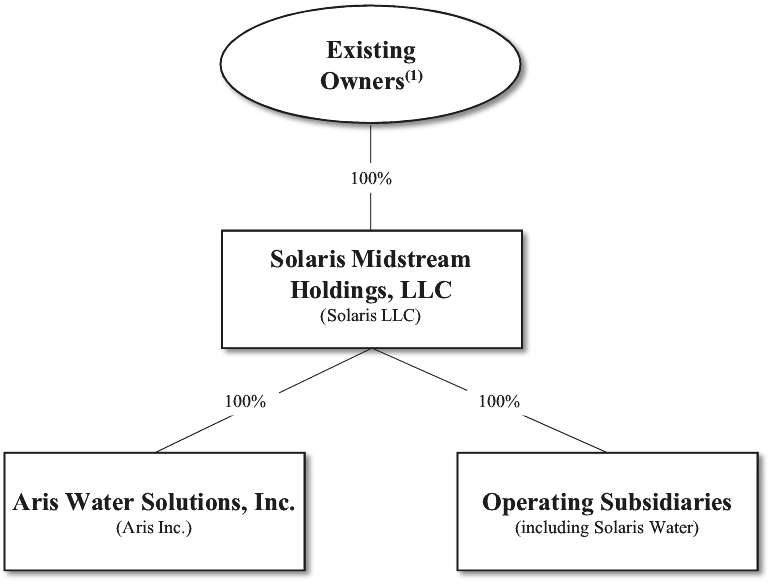
0 Response to "34 in the diagram which letter represents the transition from gas to liquid"
Post a Comment