37 co2 lewis dot diagram
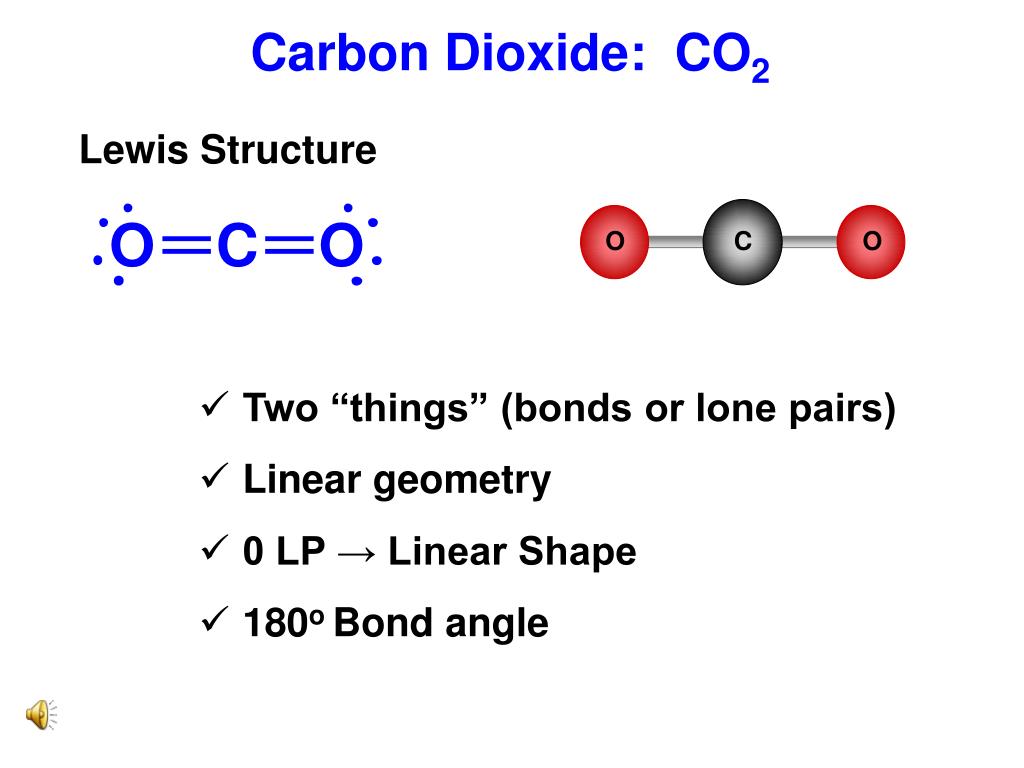
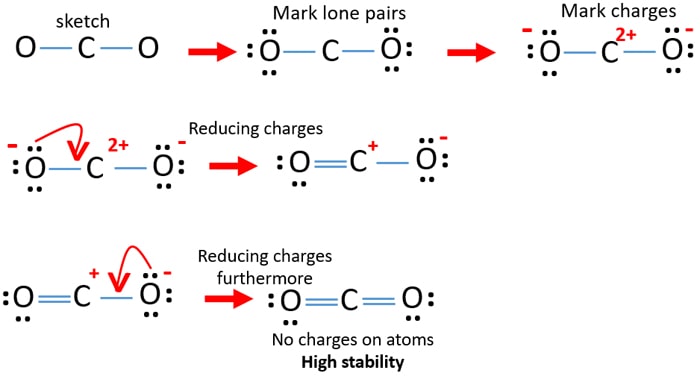
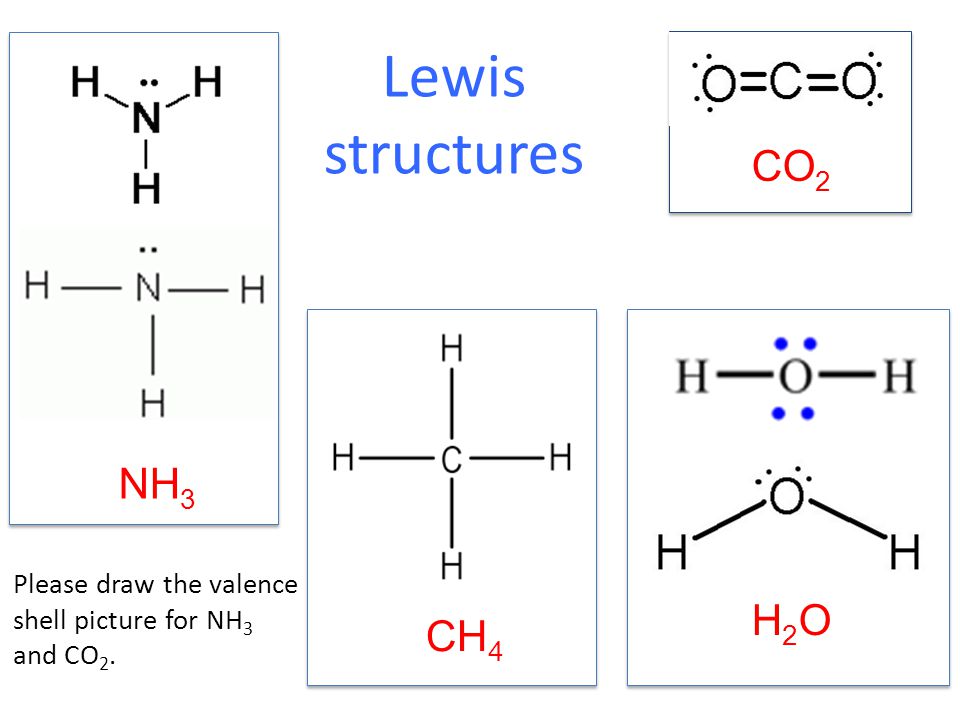
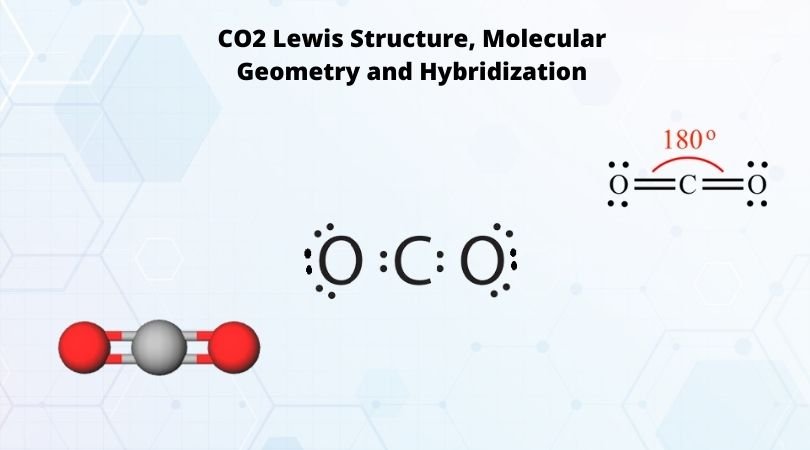
2. (Carbon dioxide) Lewis Structure and Shape. Carbon dioxide (CO 2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO 2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO 2 is linear. The Lewis structure for carbon dioxide. This diagram shows the conceptual stages of drawing the Lewis structure for a molecule of carbon dioxide (CO2). Lewis structures can also be drawn for ions. In these cases, the entire structure is placed in brackets, and the charge is written as a superscript on the upper right, outside of the bracket.
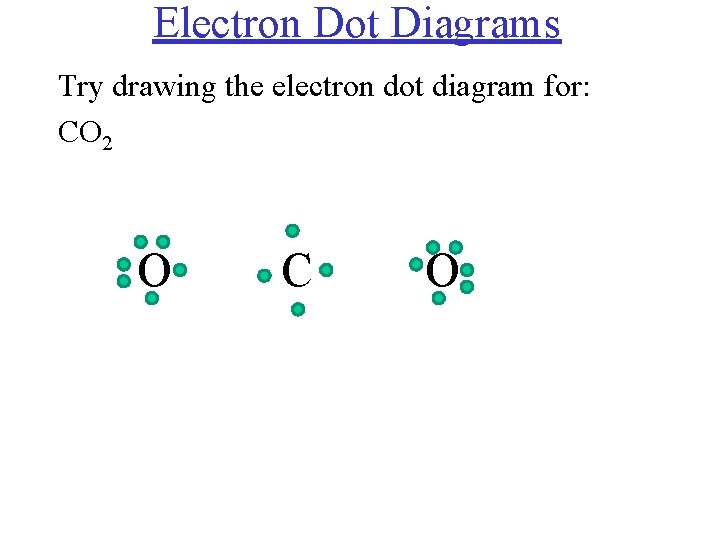
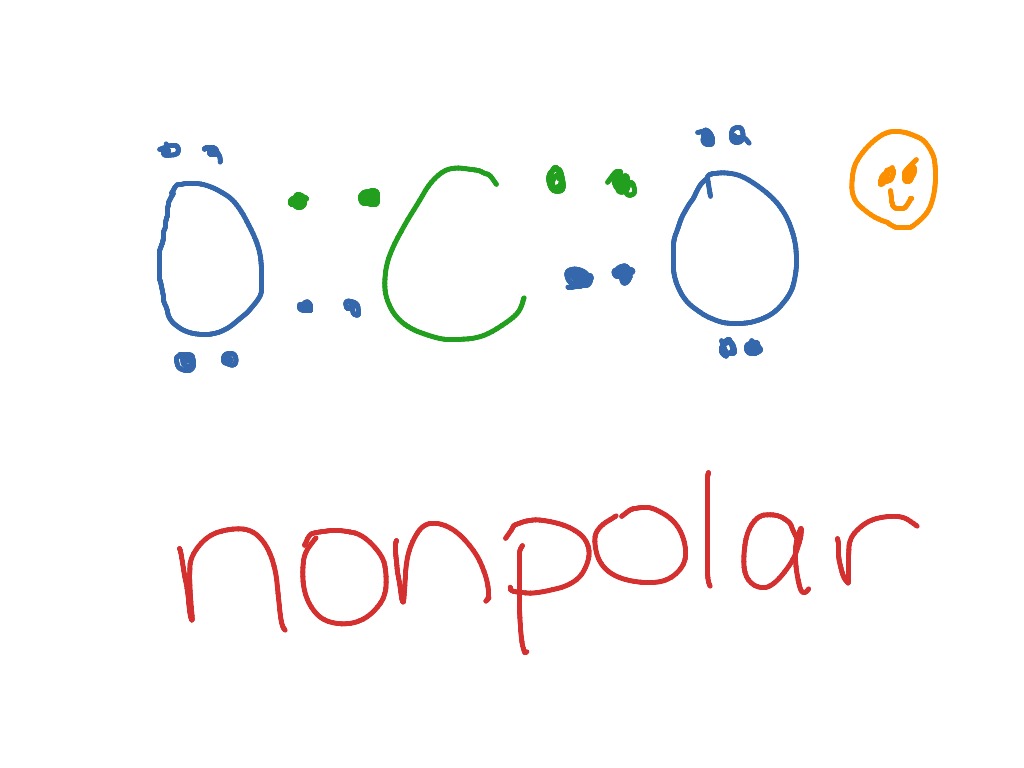
CO 2 Lewis Dot structure Is two double bonds Which Going from carbon to the oxygen atoms. In This Every Oxygen Need To bond twice and the carbon atom needs to bond 4 times. Accoridng To Octet Rule The Correct Levis Dot Structure of co2 Is :.. O = C =.. O: . Co2 Lewis Structure. Now We Draw Co2 Lewis Structure .
Co2 lewis dot diagram
CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.". Carbon dioxide (CO2) has a total of 16 valence electrons which present on the outer shell of atoms i.e. 4 carbon atoms and 12 of two oxygen atoms. From this we can easily draw the Lewis dot diagram of CO2 by adjusting two double bonds between carbon and oxygen (O=C=O). Lewis structure of carbon dioxide: This figure explains the bonding in a CO 2 molecule. Each O atom starts out with six (red) electrons and C with four (black) electrons, and each bond behind an O atom and the C atom consists of two electrons from the O and two of the four electrons from the C.
Co2 lewis dot diagram. Lewis Structures for O2. Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar - electrons are shared equally. Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen. Answer: CO2 Lewis structure (carbon dioxide electron dot structure) is that type of diagram where we show the total 16 valence electrons of CO2 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ]. Before we discuss the CO 2 lewis structure or lewis dot structure for CO2, we need to know the basics of lewis dot structure.Lewis dot structure work on the octet rule, which means that all the atoms in the structure would have eight electrons in their valence shell except hydrogen. The Lewis Dot Structure is a graphical representation of how electrons are distributed around the atoms which comprise a molecule. The reason for drawing/creating a Lewis dot structure is that it helps one predict the kinds of bonds, as well as a number of bonds, that can be formed around an atom. Lewis structures can be utilized to make ...
Carbon dioxide (CO2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO2. No lone pairs on carbon ... Which Lewis Electron-dot Diagram is Correct for Co2. co2 lewis dot structure the lewis structure of co2 looks something like that carbon c is at the center from carbon draw a two lines to each of the two oxygen o atoms surrounding it each of the two lines look like a long equal == sign each line symbolizes one bond whats the lewis dot structure for carbon dioxide co2 o c o this is the lewis ... Answer (1 of 2): CO2 Lewis Structure Answer: CO2 Lewis structure(carbon dioxide electron dot structure) is that type of diagram where we show the total 16 valence ... Covalent bond and Lewis dot structure (H2O & CO2) Google Classroom Facebook Twitter. Email. Bonding in carbon- covalent bond. Carbon and hydrocarbons. Covalent bond . Covalent bond and Lewis dot structure (H2O & CO2) This is the currently selected item. Single and multiple covalent bonds.
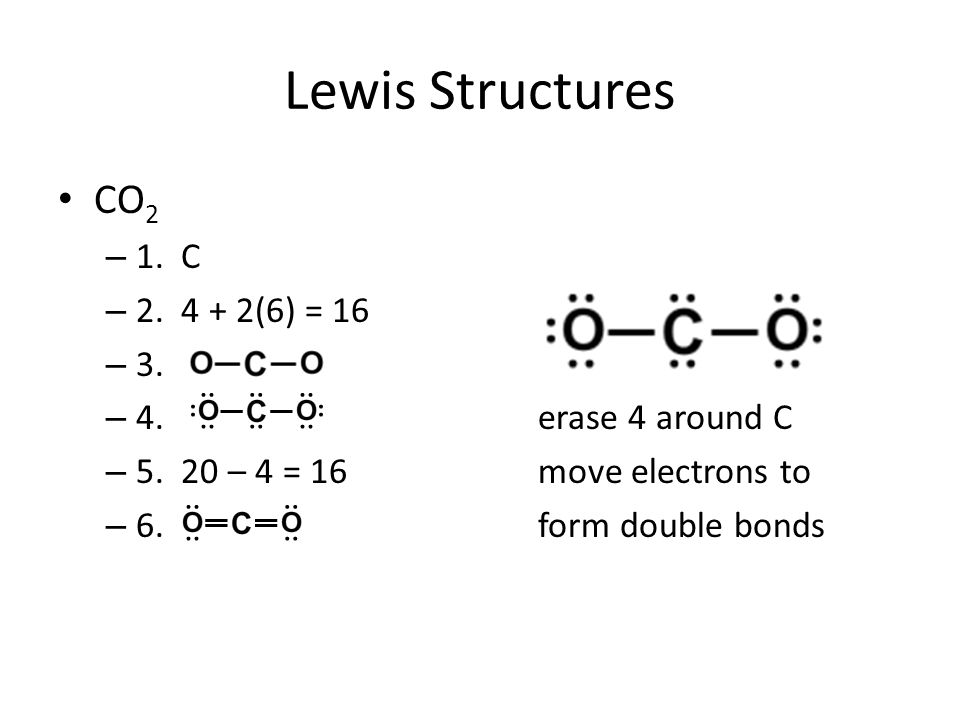
Drawing the Lewis Structure for CO 2. Viewing Notes: CO 2 is a clear, heavier-than-air gas. In the Earth's atomsphere it is considered a greenhouse gas. In the CO 2 Lewis structure carbon is the least electronegative element. Therefore it is put in the center of the dot structure. For the CO 2 Lewis structure there are a total of 16 valence ... Follow some steps for drawing the CO2 Lewis dot structure 1. Count total valence electron in CO2. In the first step, we need to calculate how many valence electrons are present in it. Valence electrons of CO2 show the outer shell electrons present around carbon and oxygen that can participate in the formation of the chemical bonds. I quickly take you through how to draw the Lewis Structure of CO2 (Carbon DiOxide). I also go over hybridization, shape and bond angles. To know the lewis structure of CO2, one should first understand what precisely the Lewis structure is. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. These valence electrons are represented by drawing dots around the individual atoms, hence the Lewis dot structure.
The Lewis Dot Structure for CO2. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for CO2. You could alternatively also draw the structure by including two dots for every bond. That would mean that you would have a total of eight dots around the carbon, thereby filling its octet. The octets of both of the oxygen atoms are also ...
How can I draw a Lewis dot diagram for carbon dioxide? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Deevona Apr 18, 2015 1. 2. Two electrons (dots) make one bond (line). Answer link. Related questions. How is the Lewis structure of an ion written? ...
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
To embed a widget in your blog's sidebar, install the Wolfram|Alpha Widget Sidebar Plugin, and copy and paste the Widget ID below into the "id" field: To add a widget to a MediaWiki site, the wiki must have the Widgets Extension installed, as well as the code for the Wolfram|Alpha widget. To include the widget in a wiki page, paste the code ...
Carbon dioxide (CO2) lewis structure has two oxygen atoms and one carbon atom. There are two double bonds around carbon atom in the CO2. No lone pairs on carbon atom and each oxygen atom has two lone pairs on their valence shells. Shape of CO2 is linear. What is the electron dot diagram of carbon dioxide? The molecular formula of carbon dioxide ...
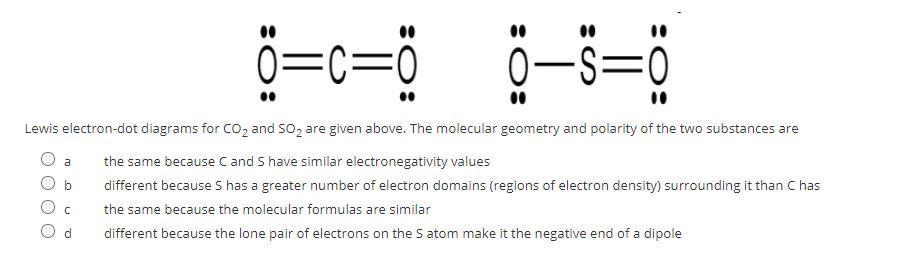
The molecular geometry can be determined from the Lewis Dot diagrams because it is determined by the number of electron domains around the central atom (Niles, 2004). Shape is determined by the number of both shared and unshared domains of electrons around the central atom and refers only to the orientation of atoms in and around the center. 4.
Lewis dot structure of atoms link. 11+ Carbon Lewis Dot Structure. However, these structures are helpful in understanding the bonding and valence electron configurations of different atoms and molecules. In the formation of co2, there are two particles; Viewing notes the carbon atom goes in the center of the lewis structure since it is the ...
Draw the dot diagram and the Lewis structure for this compound. Octet Rule: The octet rule states that atoms try to have their valence shells completely filled to increase stability.
CO Dot Structure (Key Points) Linear geometry ( linear diatomic molecule) The bond angle is 180 °C; Two lone pairs of electrons; Valence electrons of oxygen atom =6; Valene electrons of carbon atom = 4; Lewis Structure of CO 2. In the CO2 Lewis structure, there are two oxygen atoms and one carbon atom.
Lewis Dot of Carbon Dioxide. CO 2. Back. 70 More Lewis Dot Structures. Produced from the complete combustion of hydrocarbons. Used by plants during photosynthesis. CO 2 contains 2 double bonded oxygen atoms 180 o apart. This symmetrical structure gives it a nonpolar shape and weak intermolecular forces. YouTube.

What Would Be The Electron Dot Structure Of Carbon Dioxide Which Has Formula Co Sub 2 Sub Chemistry Q A
Lewis Dot structure for CO2 Setup Step-03: Now we have to determine the central atom in CO2.The central atom is that kind of atom that is single or that has lower electronegativity.In case of CO2,Carbon,C, is the central atom and oxygen ,O, is the outer atom. Lewis Dot structure for CO2 Step-04:
A step-by-step explanation of how to draw the CO2 Lewis Dot Structure (Carbon dioxide).For the CO2 structure use the periodic table to find the total number ...
Lewis structure of carbon dioxide: This figure explains the bonding in a CO 2 molecule. Each O atom starts out with six (red) electrons and C with four (black) electrons, and each bond behind an O atom and the C atom consists of two electrons from the O and two of the four electrons from the C.

Science Coverage Co2 Lewis Structure Molecular Geometry Molar Mas In 2021 Molecular Geometry Molecular Molar Mass
Carbon dioxide (CO2) has a total of 16 valence electrons which present on the outer shell of atoms i.e. 4 carbon atoms and 12 of two oxygen atoms. From this we can easily draw the Lewis dot diagram of CO2 by adjusting two double bonds between carbon and oxygen (O=C=O).
CO2 Lewis Properties. The CO 2 Lewis structure is symmetric. Generally, small symmetric molecules are nonpolar. CO 2 is a nonpolar substance, meaning it tends to be a gas. CO 2 has a rather low boiling point of around -80 ℃ or -100 ℉. It can be liquified and even frozen solid with special machinery to produce "dry ice.".



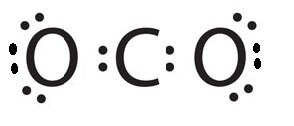


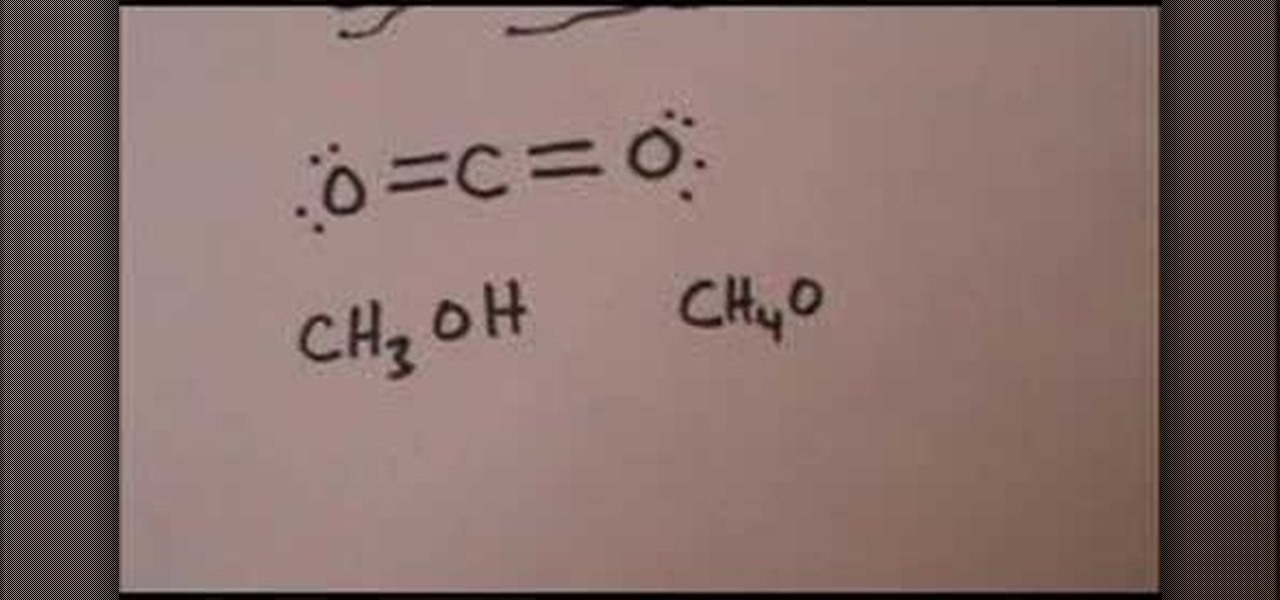





:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)







/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)

0 Response to "37 co2 lewis dot diagram"
Post a Comment