34 octahedral molecular orbital diagram
Molecular orbital diagram corresponding to an octahedral MX N complex. Atomic levels of the transition-metal impurity, M, (ligand anions, X) are depicted on the left-hand (right-hand) side of the ... New bifunctional TCNQ-based material · New bifunctional TCNQ based material: [Co(terpy)2](TCNQ)3·CH3CN exhibits a high room temperature conductivity of 0.13 S cm−1 and an anomaly in conductivity at ∼190 K as evidenced by variable temperature structural, magnetic and conductivity studies
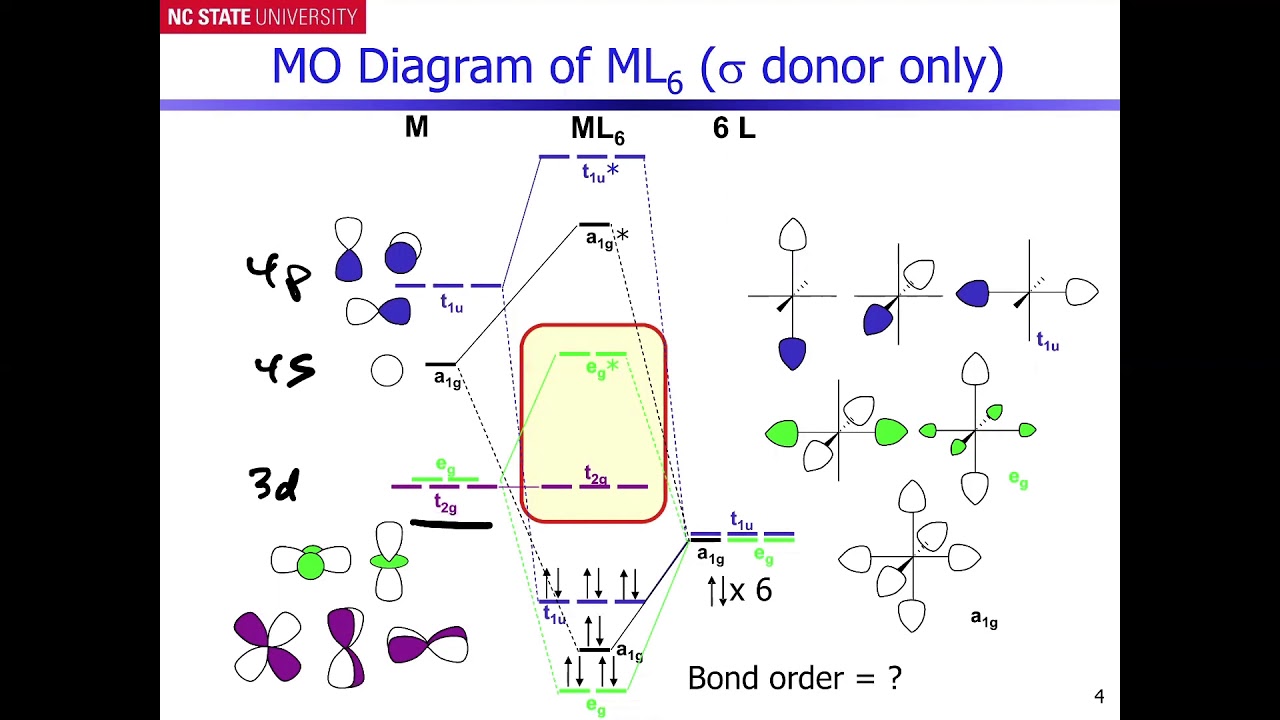
Show below is a molecular orbital diagram for an octahedral ML 6 complex and the O h character table. From the ligand pi orbitals, only the t 2g set is considered. All ligand orbitals are fully filled. Guiding lines connecting atomic orbitals to their corresponding molecular orbitals have been omitted.

Octahedral molecular orbital diagram
In about 15 minutes animated video, the concept of Molecular Orbital Theory (MOT), symmetry of metal atomic orbitals in different ligand environment and mole... If the central atom possesses partially occupied d-orbitals, it may be able to accommodate five or six electron pairs, forming what is sometimes called an "expanded octet." Molecular Geometries. Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the VSEPR theory. August 14, 2020 - The characteristics of transition metal-ligand bonds become clear by an analysis of the molecular orbitals of a 3d metal coordinated by six identical ligands in octahedral complexes [ML6]. As the …
Octahedral molecular orbital diagram. Metal atomic orbitals Molecular orbital diagram of weak complex [CoF 6]3-Total 12 e will be contributed as there are six ligands in an octahedral complex Co3+ = d6 So total electrons = 6 (Metal) + 12 (Ligands) = 18 4 unpair electrons, therefore paramagnetic Atomic orbital (Metal) (Molecular orbitals) Atomic orbital (Ligands) σ-bonding (sigma bonding) In an octahedral complex, the molecular orbitals created by coordination can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal.In octahedral complexes, ligands approach along the x-, y- and z-axes, so their σ-symmetry orbitals form bonding and anti-bonding combinations with the d z 2 and d x 2 −y ... d orbitals •l = 2, so there are 2l + 1 = 5 d-orbitals per shell, enough room for 10 electrons. •This is why there are 10 elements in each row of the d-block. σ‐MOs for Octahedral Complexes 1. Point group Oh 2. The six ligands can interact with the metal in a sigma or pi fashion. Let’s consider only sigma interactions for now. with five molecular orbitals, five atomic orbitals are mixed: ... In molecules This will give trigonal bipyramidal geometry and is called dsp3 hybridization. molecular orbitals. Finally, molecules with octahedral geometry, will have This hybridization is called Shown below is a portion of the chart from Worksheet 14. Fill in the
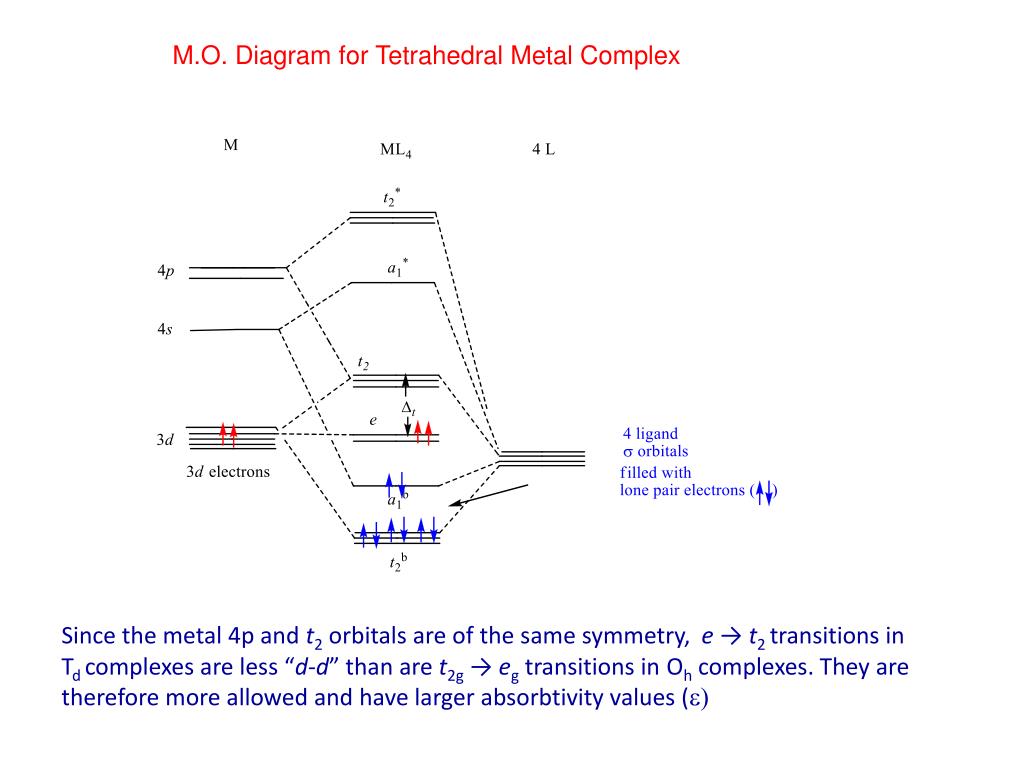
High‐Spin and Low‐Spin Configurations • In an octahedral complex, electrons fill the t 2g and e g orbitals in an aufbau manner, but for configurations d4 - d7 there are two possible fillingschemesdependingon the magnitudeof o. • The relative magnitudes of o and the mean pairing energy, P, determine whether a high spin or low spinstate isobserved inoctahedralcomplexes. The tetrahedral MO diagram is similar to the octahedral molecular orbital diagram. On the left, the metal ion orbitals are present, and on the right side are the ligand orbitals. The center depicts the orbitals of the metal complex. There are four ligands in tetrahedral complexes. November 22, 2019 - Self-consistent charge and configuration (SCCC) molecular orbital calculations are reported for 32 selected octahedral and tetrahedral first-row transition-metal complexes containing halide and chalcogenide ligands. It is found that for the range of metal oxidation states II through IV, Fsigma, ... Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
In this video I have explained SALC (Symmetry adopted linear combination) of transition metal valence orbitals and LGO'S (ligand group orbitals) to construct... The original version of this website (2003) was created back when I was at University, as a place to store my Chemistry notes for others - particularly later year groups - to have a little bit of help if they need it. I've recently (2019) brought the website up to date as it was showing serious ... #bondingincoordinationcompounds #octahedralcomplex Guiding fines connecting atomic orbitals to their corresponding molecular orbitals have been omitted. Answer the following questions using; Question: Shown below is a molecular orbital diagram for an octahedral ML. complex and the character table. Assume there are no ligand orbitals with pi symmetry that interact with the metal.

Lecture 26 Mo S Of Coordination Compounds Mlx X 4 6 1 Octahedral Complexes With M L S Bonds Only Consider An Example Of An Octahedral Complex Ppt Video Online Download
Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis
00:21 Molecular orbital diagram for tetrahedral complex03:36 Molecular orbital diagram for octahedral complex"Kim Method" - MO diagrams for tetrahedral a...
Ppt Molecular Orbital Theory Approach To Bonding In Transition Metal Complexes Powerpoint Presentation Id 5491714
In chemistry, octahedral molecular geometry describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron.The octahedron has eight faces, hence the prefix octa.The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between ...
1. Molecular orbital theory and the octahedron 2. The MO energy diagram and Δo 1. Octahedral transition metal complexes utilize s, p and d-orbitals in their bonding. For a first row transition metal, these are the 3d, 4s and 4p orbitals (the valence orbitals). Here we will create a molecular
Molecular orbital theory Octahedral complexesDiagrams with examplesExplainedThanks for watching!!!!!#chemistry
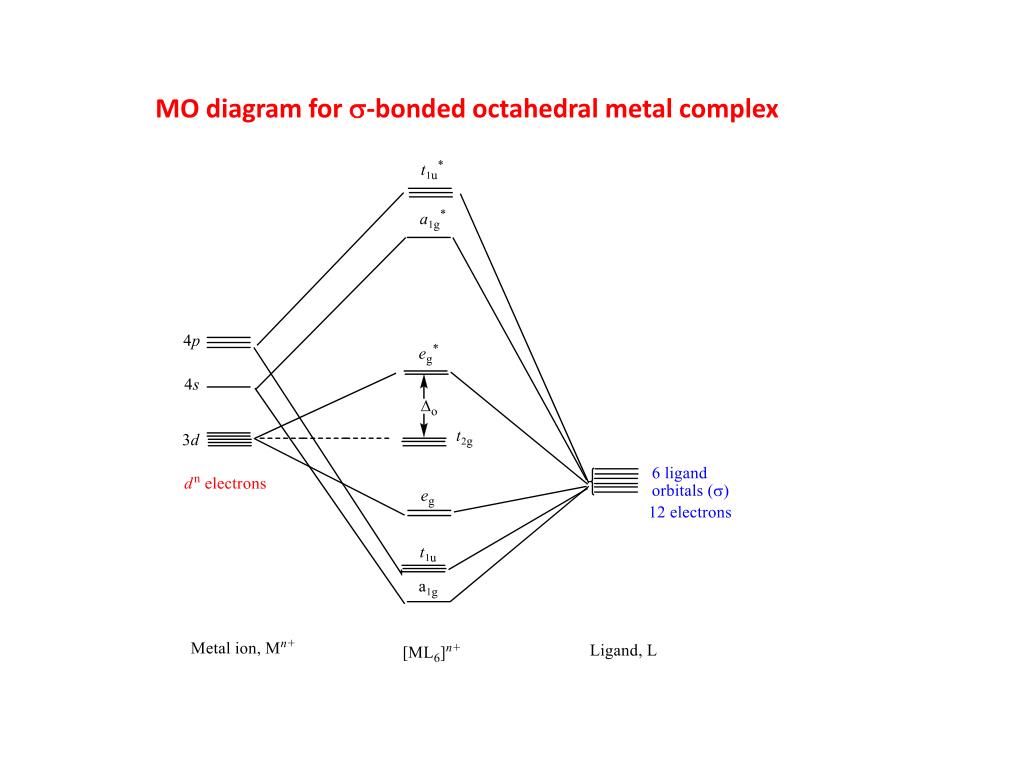
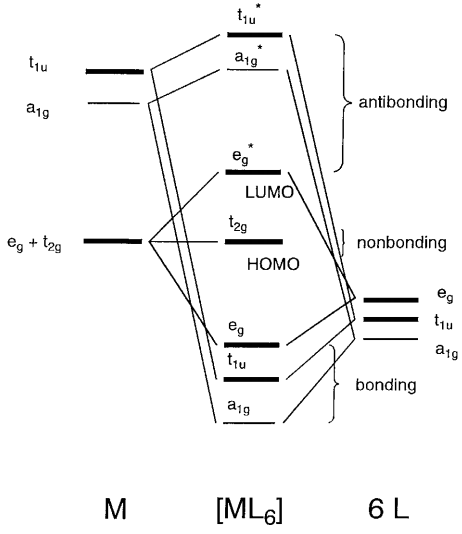
Ligand Field Theory The molecular orbital diagram is consistent with the crystal field approach. Note that the t2g set of orbitals is non-bonding, and the eg set of orbitals is antibonding. 27. Ligand Field Theory The electrons from the ligands (12 electrons from 6 ligands in octahedral complexes) will fill the lower bonding orbitals. { 28.
Wolfsberg-Helmholtz molecular orbital calculations on an octahedral cluster of cobalt atoms have suggested that the 86 valence electrons in the carbonyl anion Co 6 (CO) 14 4- are all accommodated in bonding and weakly antibonding molecular orbitals and consequently represent a stable closed-shell electronic configuration. The 11 strongly antibonding skeletal molecular orbitals in such ...
Please use Internet Explorer web browser for site best experience or Click here · e-PG Pathshala is an initiative of the MHRD under its National Mission on Education through ICT (NME-ICT) being executed by the UGC. The content and its quality being the key component of education
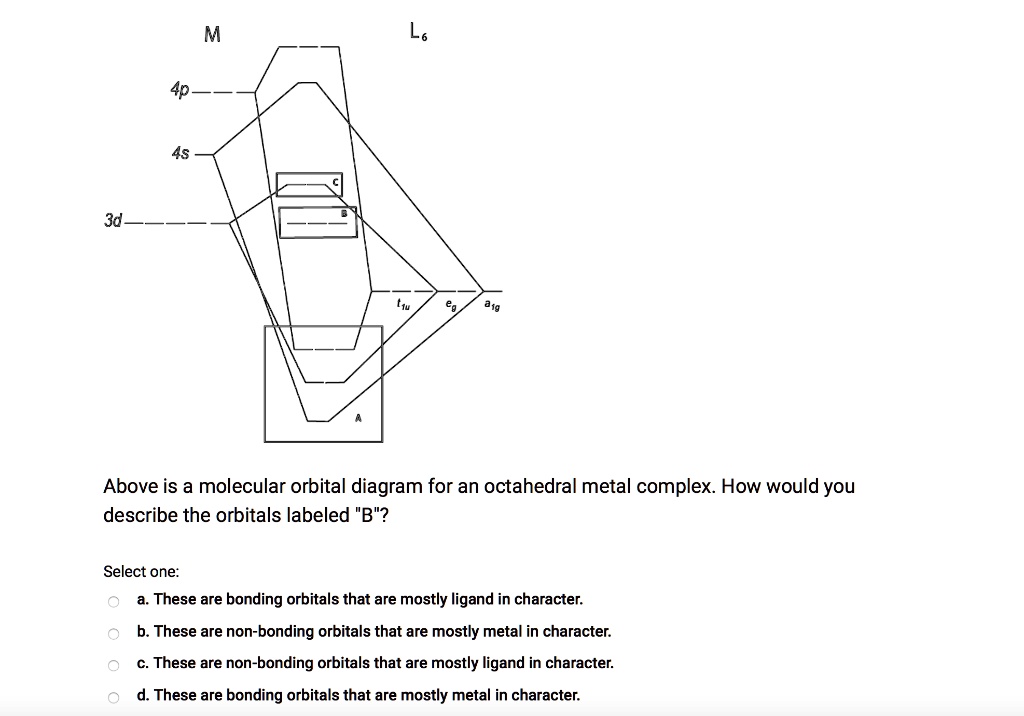
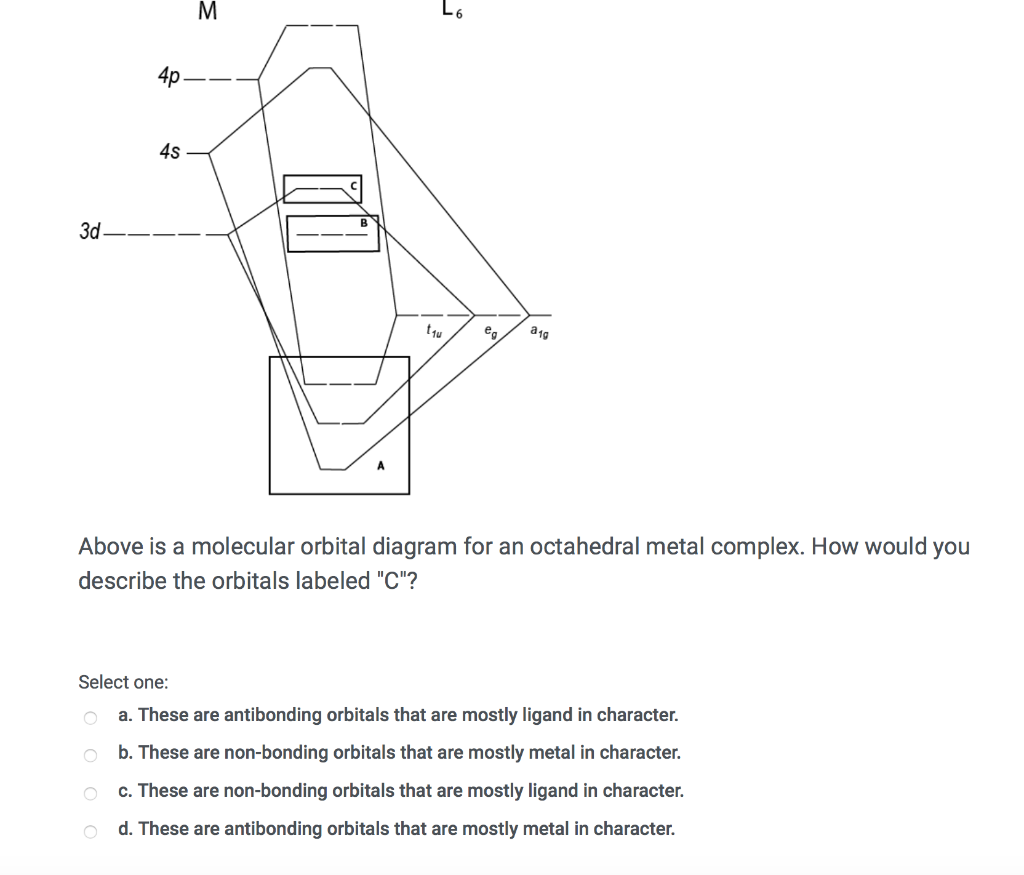
Solved M 97 4p 4s 3d Above Is A Molecular Orbital Diagram For An Octahedral Metal Complex How Would You Describe The Orbitals Labeled B Select One These Are Bonding Orbitals That Are
Self‐consistent charge and configuration (SCCC) molecular orbital calculations are reported for 32 selected octahedral and tetrahedral first‐row transition‐metal complexes containing halide and chalcogenide ligands. It is found that for the range of metal oxidation states II through IV, F σ, chosen to fit the experimental Δ, is a function of only the metal atomic number for constant F π.
Download "Molecular Orbital Theory - Octahedral, Tetrahedral or Square Planar Complexes" ATOICV1-7-2-Molecular-Orbital-Theory-Octahedral-Tetrahedral-or-Square-Planar-Complexes.pdf - Downloaded 87 times - 834 KB. Share this article/info with your classmates/friends and help them to succeed in their exams.
Ppt Molecular Orbital Theory Approach To Bonding In Transition Metal Complexes Powerpoint Presentation Id 6714303
Molecular Orbital Approach to Coordination complexes - YouTube
Draw the molecular orbital diagram shown to determine which of the following is most stable. O22+ ... Identify the number of electron groups around a molecule with an : octahedral shape. 6. Give the approximate bond angle for a molecule with an : octahedral shape. 90. The bond angle in NH3 is. 107. Give the major force between acetone and ...
Prof. Marcetta Y. Darensbourg myd@mail.chem.tamu.edu 408 Chemistry Bldg. (979) 845-5417 Office Hours: Th 11-12; M 11-12. Or any afternoon · Admin. Coord.: Abbey Kunkle darensbourg_asst@chem.tamu.edu 407 Chemistry Bldg. (979) 845-5417
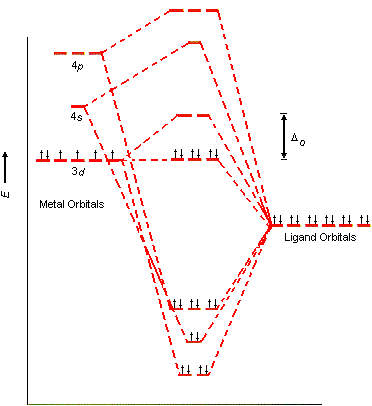
Molecular orbital diagram for a simple octahedral complex. This is qualitatively equivalent to the results of the simpler crystal field theory, which ignores orbital overlap with the ligand orbitals and uses just the electrostatic repulsion of the metal d-orbitals and the negative charges of the ligands.
May 7, 2021 - Figure \(\PageIndex{5}\): (a) Tetraheral ligand field surrounding a central transition metal (blue sphere). (b) Splitting of the degenerate d-orbitals (without a ligand field) due to an octahedral ligand field (left diagram) and the tetrahedral field (right diagram).

Scielo Brasil A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate Chemistry Students A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate
Molecular orbitals for Octahedral complexes The combination of the ligand and metal orbitals (4s, 4p x, 4p y, 4p z, 3d z2, and 3d x2-y2) form six bonding and six antibonding with a 1g, e g, t 1u symmetries. The metal T 2g orbitals do not have appropriate symmetry - nonbonding Electron in bonding orbitals provide the potential energy that holds ...
A molecular orbital diagram which estimates the energies of the bonding (show above) antibonding and non-bonding orbitals is shown below. Since there is a large disparity in energy between the ligand orbitals and the metal orbitals, the lower lying molecular orbitals in the diagram are essentially ligand orbitals.
Give the approximate bond angle for a molecule with an octahedral shape. A) 109.5° ... Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable.
September 16, 2020 - Thus, the electrons can fill the lowest energy molecular orbitals available to them. However, the electron pairing may be different if the electrons were allowed to fill the lowest energy atomic orbitals available to them. ... This diagram shows the field splitting of a metal with ligands in an octahedral ...
Fig. 2. Simplified molecular orbital diagram for a low spia octahedral complex, such as [Co(NH3 )g, where A = energy difference a, e, and t may be antisymmetric (subscript ungerade) or centrosymmetric (subscript, gerade) symmetry orbitals.
The three lower-energy orbitals ... labels are based on the theory of molecular symmetry: they are the names of irreducible representations of the octahedral point group, Oh.(see the Oh character table) Typical orbital energy diagrams are given below in the section High-spin ...

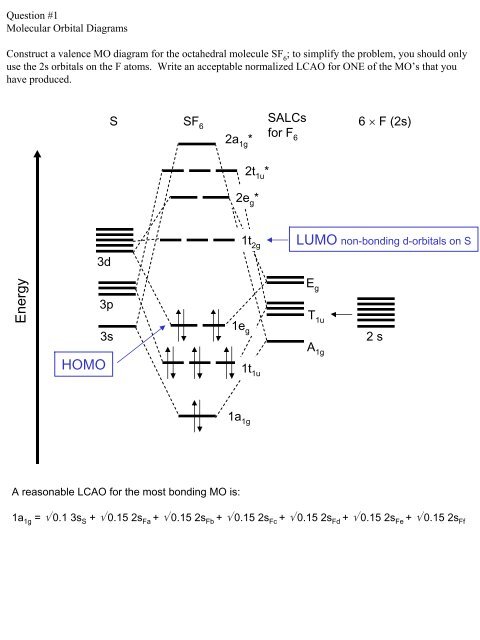
1 Draw The Molecular Orbital Diagram Of Transition Metal Ion In High Spin Mn H2o 4 Oh 2 Complex Also Determine Homeworklib
Molecular orbitals diagrams for octahedral coordination compound without pi interactions. 1. Molecular orbital Diagram for ML6 Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa. 2. nd Six donor orbitals (n-1)s (n-1)p Δo Metal atomic orbitals [ML6] molecular orbitals M. O. diagram for [ML6] complex (no π ...
a) The molecular orbitals of octahedral first row d-metal complexes [ML6] (in which there is no pi bonding) have the following order of descending energy: t1u > a1g > eg > t2g > eg > t1u > a1g. Construct a molecular orbital energy level diagram showing the energies of the molecular orbitals of the complex [ML6] and the energies and identities ...
The orbitals responsible for -bonding are not shown. The energy scale is expanded relative to the -complex MO diagram. Determine which diagram is for the -donor and -acceptor cases. Label the MOs with the appropriate symmetry labels and the o. ... Molecular Orbital Theory of Octahedral Complexes ...

The Electronic Spectra Of Alkali Metal Uranates And Band Assignments An Analysis Of Their Diffuse Reflectance Spectra Physical Chemistry Chemical Physics Rsc Publishing
In octahedral complexes, the molecular orbitals created by the coordination of metal center can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal. The metal orbitals taking part in this type of bonding are nd, (n+1)p and (n+1)s. It should be noted down
In chemistry, octahedral molecular geometry describes a molecular geometry in which 6 ligands are symmetrically arranged around a central atom in an octahedral geometry. All six ligands are chemically equivalent. The central atom is often a transition metal.The octahedron has eight faces (hence the prefix octa) and thus is one of the Platonic solids (although octahedral molecules typically ...
February 3, 2021 - 20.4: Molecular Orbital Theory - Octahedral Complexes
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
August 14, 2020 - The characteristics of transition metal-ligand bonds become clear by an analysis of the molecular orbitals of a 3d metal coordinated by six identical ligands in octahedral complexes [ML6]. As the …
If the central atom possesses partially occupied d-orbitals, it may be able to accommodate five or six electron pairs, forming what is sometimes called an "expanded octet." Molecular Geometries. Molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the VSEPR theory.
Solved 2 A Molecular Orbital Diagram For A Tetrahedral Transition Metal Complex Is Shown Below Show How The Electrons Occupy The Molecular Orbita Course Hero
In about 15 minutes animated video, the concept of Molecular Orbital Theory (MOT), symmetry of metal atomic orbitals in different ligand environment and mole...











0 Response to "34 octahedral molecular orbital diagram"
Post a Comment