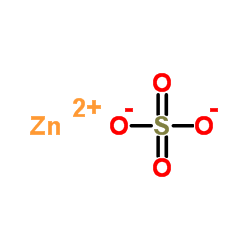
37 lewis dot diagram for zinc
Zinc: Iodide: Zinc iodide (ZnI2): 51 Lewis Dot Diagrams for Compounds Pre-work video for visual learning if baseline expectations were met. Classwork: In the Prentice Hall workbook (red & blue) complete Section 7.2, pg 61-62 HOMEWORK: In Pearson Chemistry textbook, read pages , then... Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. What column of the periodic table has Lewis electron dot diagrams that have six electrons in them? Draw the Lewis electron dot diagram for each element.
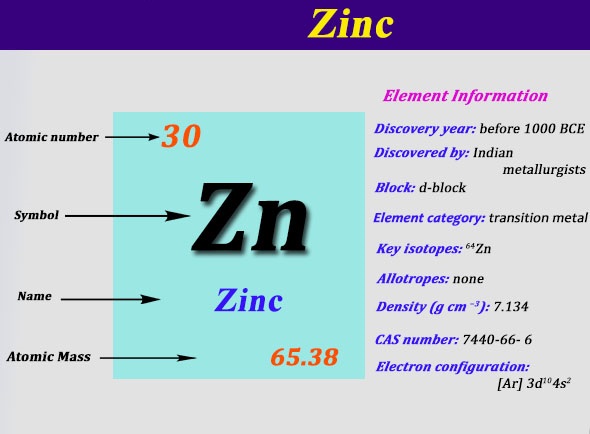
Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. Zinc (element number 30) is in the 4th Period of the Periodic Table. From left to right, you count two #"4s"# electrons and ten #"3d"# electrons.

Lewis dot diagram for zinc
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Explain why the first two dots in a Lewis electron dot diagram are drawn on the... • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. • Use valence shell electron pair repulsion (VSEPR) model to draw and name molecular shapes (bent, linear, trigonal planar, tetrahedral, and trigonal pyramidal). Lewis electron dot diagram (5.3) A pictorial model of an atom, molecule, or ion consisting of the symbol(s) for element(s) and dots representing the valence electrons. Draw a Lewis electron-dot diagram for the cyclosilicate ion (SigOis ), which forms part of the structures of beryl and emerald.
Lewis dot diagram for zinc. Recently we began a chapter in Chemistry where we had to find the Lewis Dot Structures of Molecules (I.e. using valence electrons, adding in bonds, lone pairs, etc.) and I simply cannot get it. The process and sequence to get to the finished product is confusing, which is unfamiliar to me, as I am someone who typically very easily grasps concepts. I would very much appreciate anyone who could shed some light on their ways of thinking, easier way to go about it, or other tips, as my teacher has e... A step-by-step explanation of how to draw the Zn Lewis Dot Structure.For the Zn structure use the periodic table to find the total number of valence... You write Zn and then you put one dot on top (or bottom) and one on the left (or right) like this for example: Zn . . (the reason why you have 2 dots is because zinc has only two valence electrons). Cu ii lewis dot Dot structure for cooper Copper sulfate pentahydrate dot structure Electron dot structure of cuso4. source: What is the chemical equation of zinc added to copper ii sulfate and the products? Diagram showing how copper immersed in copper 2 sulphate can be measured.
A Lewis structure (also called Lewis dot formulas, Lewis dot structures, or electron dot structures) are pictorial diagrams that represent the bonding Lewis diagrams contain 3 basic elements: symbols that represent individual atoms, dots that represent electrons, and unbroken lines that represent... 2. FORMAL CHARGE AND LEWIS DOT DIAGRAMS Draw the Lewis Dot Diagram for SCN- - S C N 6 + 4 + 5 + 1 = 16 electrons But this isn't the only possible 10. FORMAL CHARGE AND LEWIS DOT DIAGRAMS Furthermore, formal charges can be used to identify which part of a molecule is charged... If a Lewis dot diagram for nitrogen shows it has 5 electrons, how many places does it have for other elements to attach? is Bromine Zincide the correct name for the formula made between Bromine and Zinc? no, it's Zinc bromide. metals have __ oxidation numbers because they can lose electrons. Unit 8: Drawing Molecules - Arlington Independent School District Unit 8: Drawing Molecules . Objectives draw the Lewis dot diagram. Aluminum Al In ionic compounds, the cation gives electron(s) to the anion.
This chapter will explore yet another shorthand method of representing the valence electrons. The method explored in this lesson will be a visual representation of the valence electrons. We will, as we observed in the previous lesson... Hello! Can someone please explain to me how to draw the Lewis diagrams for transition metals? I understand how to find the valence electrons based on the electron configuration. For elements 27+, they begin having more than 8 valence? Please help I am confused! pentabromide dinitrogen monoxide TODAY: read page 37 and pages 164167 Diatomic molecules Lewis Dot Diagrams for Molecules HW: Worksheet N2 O2 H2 Cl2 I2 F2 Br2 or Br2 I2 N2 Cl2 H2 O2 F2 show the valence electrons as dots inner electrons and atomic nuclei are represented by the... 1. Draw the Lewis dot structure for each atom of the compound to show how many valence electrons are present in each atom. The Lewis structure shows the calcium with no dots (electrons), and the chlorine ions with a complete octet. Notice the placement of the charge notation on the ions.
6.1 Lewis Electron Dot Diagrams. Learning Objectives. By the end of this section, you will be able to A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of...
Draw a Lewis electron dot diagram for an atom. Know the importance of Lewis dot in bonding. To write an element's Lewis dot symbol, we place dots representing its valence electrons, one at a time, around the element's chemical symbol.
Lewis dot structures help predict molecular geometry. Lewis structures first came into use early in the twentieth century when chemical bonding was poorly understood. Electron dot diagrams help illustrate electronic structure of molecules and chemical reactivity.
lewis dot diagram for sodium hypochlorite
Hi, I am working on a project in which we need to draw the Lewis Dot Structure for five binary molecular compounds. Although (I think) I understand how to do these with normal elements, I am confused on how to do it with compounds. The teacher has allowed us to search these up (it is one small part of the project), but I am unable to find or understand what comes up. ​ Examples: Triphosphorus pentanitride (P3N5) Searching up brings up images that contain more than 3/5 of each el...
Shouldn't oxygen have two bonds? or does the negative outside the brackets signify that both oxygen and hydrogen gain that one electron? Am I thinking about this properly?!
Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students. Electrons in the Lewis Structure (electron dot diagram) are paired to show the bonding pair of electrons.
A Lewis structure or Lewis dot diagram, represents the bonds formed between two non-metal atoms as they share electrons. These diagrams show only the valence electrons of each atom as they are distributed amongst the bonded atoms. Drawing such diagrams is a great start to understanding how...
If you have a link or image of one you can send to me that would be appreciated!
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. NOTE: On the Internet, OGT and some textbooks, the Lewis Dot Diagrams are not drawn properly. For example, carbon is drawn
Lewis Structure For Zinc Bromide Dot Diagram For Zinc Bromide Valence Electrons For Znbr2 Ls-Ionic.
Drawing Lewis dot structures (also known as Lewis structures or Lewis diagrams) can be confusing, particularly for a beginning chemistry student. For example, N2 (nitrogen gas) has a triple bond connecting the 2 nitrogen atoms. So, its bond will be notated in a Lewis diagram as 3 parallel lines...
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
Lewis Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. Lewis Dot Diagrams are illustrations of how the elements in a covalent bond come together to form a new, a structure or a molecule.
If anyone can give me an explanation or help me understand how it will look.
Draw Lewis dot structures for each of the following atoms Carbide ion Bromide ion Zinc ion Barium ion. Predict the common oxidation numbers (CHARGE) for each of the following elements when they form ions.
A Lewis Structure is a diagram that shows how valence electrons within a compound are distributed among its atoms. Those electrons that are shared by two Those electons that are located on a single atom are referred to as lone pairs and represented by two dots. Thinking About Lewis Structures.
I am having trouble with a question about a lewis dot diagram. If phosphorus and bromine atoms formed a compound, what would the lewis dot diagram and chemical formula be? Would the chemical formula be: PBr? (I couldn't find the formula anywhere on the internet to confirm if the formula is simply PBr). If it is not simply PBr, why or what makes it something else? If it is just PBr, would the lewis diagram be a single bond? \-I think I did it correctly, just wanting to make sure.
Could some one please explain to me why [this](http://www.wolframalpha.com/input/?i=Thiocyanate&a=*C.Thiocyanate-_*ChemicalIntermediate-) sulfur has a negative charge? Thank You.
A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion.
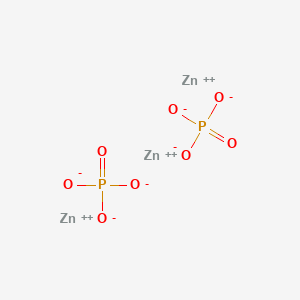
Lewis Diagram Iron Free Wiring Diagram For You. Lewis Dot Diagram And Octet Rule Youtube 5295314400801 Iron. Solved I M Totally Overwhelmed Trying To Complete This. Lewis Dot Diagram For Zinc Oxide Creativehobby Store. Complex Ions. Iron Dot Diagram Schematic Diagram.
**A Savage Christmas Eve With Lewis** It's a savage, savage Christmas, and a .... oh you know what, screw it. Rickett and his gang of hoodlums are going to ruin Christmas for everyone! Edit: **NOT A GIVEAWAY, JUST SELLING** [https://crypto.com/nft/profile?asset=10f9d19501e06ec2c057bb24322a3c4f&edition=08d4ea12baa3c5a5f34fdb3fc7c8fc7d&detail-page=PROFILE&event=theheavymachine](https://crypto.com/nft/profile?asset=10f9d19501e06ec2c057bb24322a3c4f&edition=08d4ea12baa3c5a5f34fdb3f...
Lewis electron dot diagram (5.3) A pictorial model of an atom, molecule, or ion consisting of the symbol(s) for element(s) and dots representing the valence electrons. Draw a Lewis electron-dot diagram for the cyclosilicate ion (SigOis ), which forms part of the structures of beryl and emerald.
• Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. • Use valence shell electron pair repulsion (VSEPR) model to draw and name molecular shapes (bent, linear, trigonal planar, tetrahedral, and trigonal pyramidal).
Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Explain why the first two dots in a Lewis electron dot diagram are drawn on the...


















:max_bytes(150000):strip_icc()/Europium-58b6016e3df78cdcd83cdb23.jpg)



0 Response to "37 lewis dot diagram for zinc"
Post a Comment