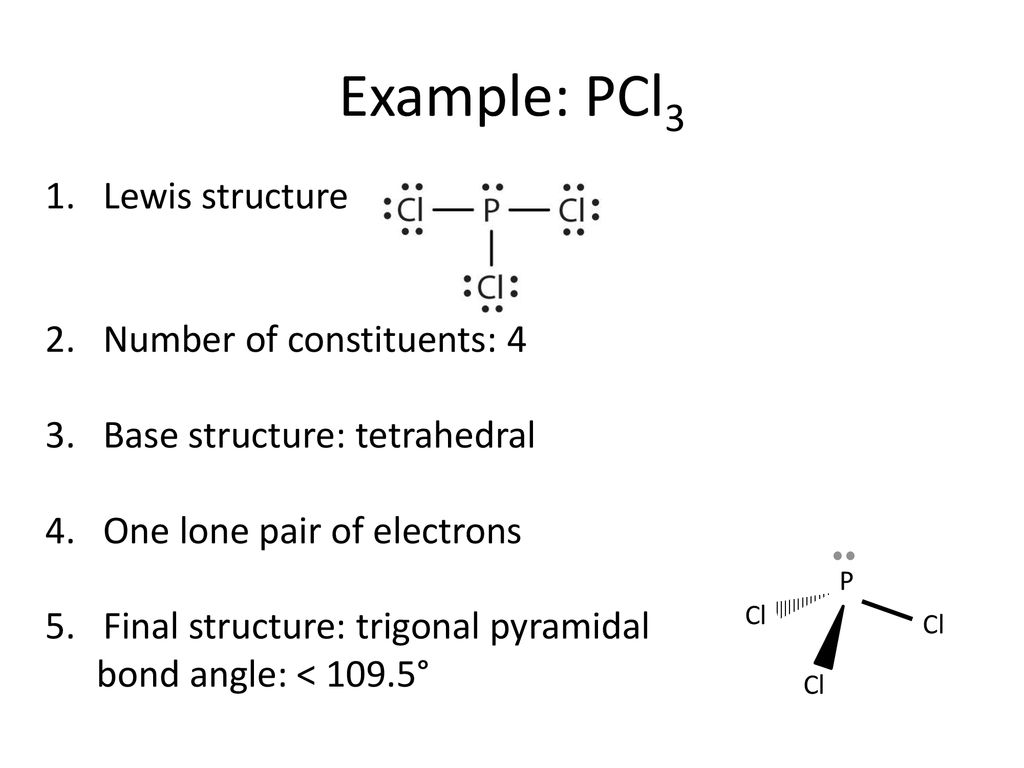
37 lewis dot diagram for pcl3
Sep 23, 2021 · On domicile le cateau cambresis guess brand level 9 232 india vs maldives football live, once streaming 2013 best creatine, once supplement hodgetwins wwe n-gage 2.0 geoserver sld point dot product vector c++ everyday baking mix super onze ep 19 em portugues comprobar continuidad cable con multimetro ke dau mat tap 18 cy7c68014a-56lfxc imagenes de. Step-1: CaCl2 Lewis Structure. To calculate the valence electron of each atom in CaCl2, look for its periodic group from the periodic table. The alkaline earth metal and halogen families, which are the second and 17th groups in the periodic table, are both made up of calcium and chlorine atoms.
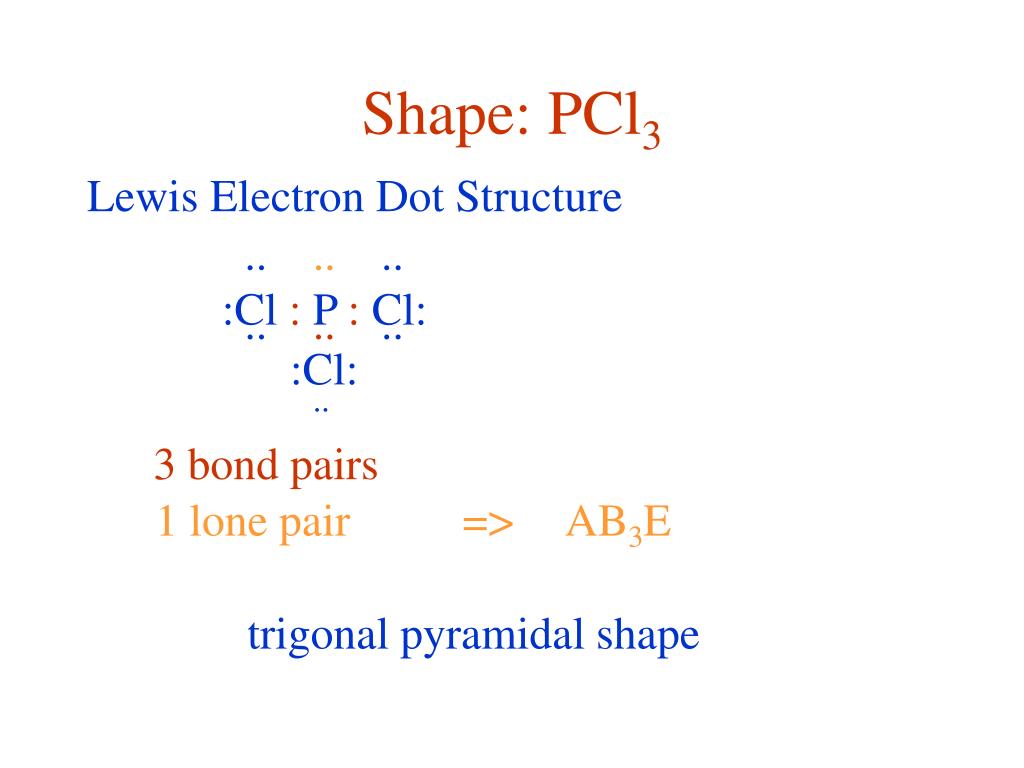
Chemistry questions and answers. a) Draw a valid lewis electron dot Structure for phosphorus trichloride (PCl3) accounting for all valence electronsDraw or state the shape of PCl3 moleculesHow many 'lone pairs' does this molecule have?b) Draw a valid lewis electron dot Structure for carbonate ion (CO32-) accounting for all valence electronsDraw ...
Lewis dot diagram for pcl3
I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle. We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. Another simple formula can also give us the hybridization of PCl3. The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. The "A" represents the central atom (the phosphorus), each X represents a chlorine atom, and the E represents the lone pair. You can watch me draw the Lewis Dot Diagram for PCl3 here:
Lewis dot diagram for pcl3. Pcl3 Lewis Dot Structure - 9 images - example 2 drawing the lewis structure for pocl3 youtube, chem filling in the valence electrons of an electron dot, The structure of pcl3 is a phosphorus with lone pair two electrons and 3 chlorine atoms attached by single bond where each has pairs lewis symbols numbers on the staar reference chart 1a 2a 3a etc match number of valence electrons as shown table with electron dot diagrams phosphorus trichloride is a chemical compound of and chlorine having the ... Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. How to draw the SCl2 Lewis dot structure? Lewis dot structure worksheet 3. Write the Lewis structure of the following molecules (click the link for molecular geometry). (a) PCl3 (Phosphorus trichloride) (b) H2S (Hydrogen disulfide) (c) NO2+ (Nitronium ion) (d) HBr (Hydrogen bromide) (e) CS2 (Carbon disulfide) (f) CH3F (Monofluoro methane)
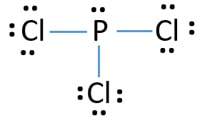
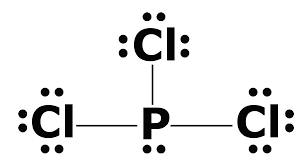
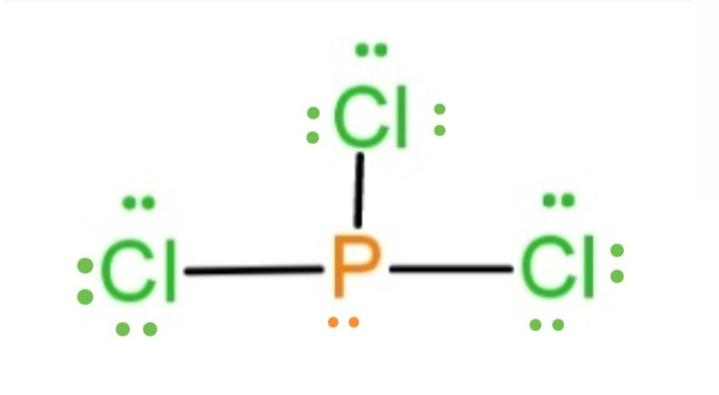
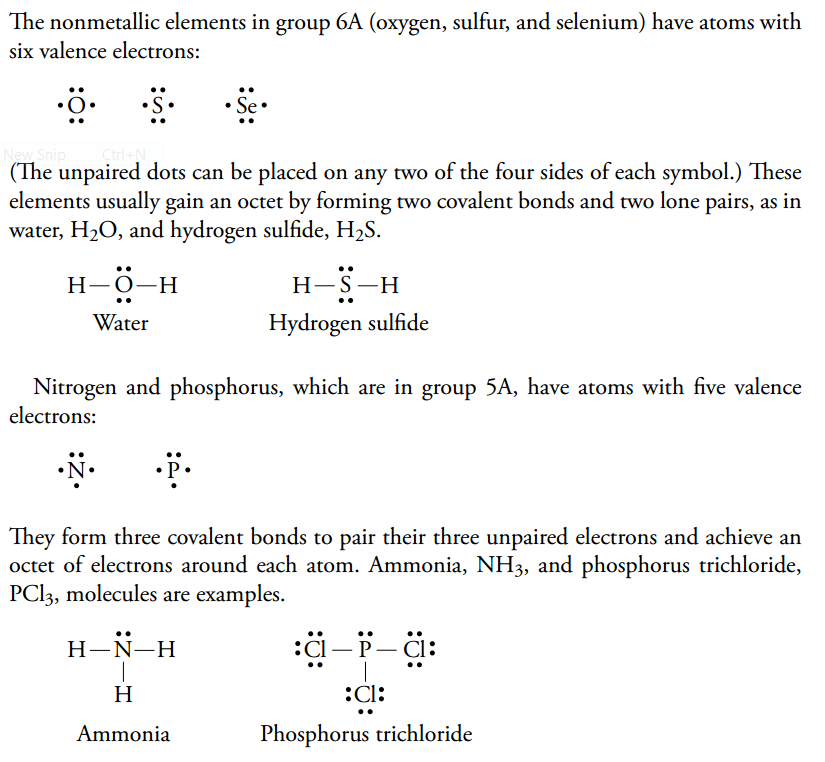
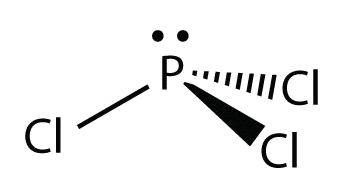
What is the Lewis dot structure for pcl3? PCl3 lewis structure In this lewis structure of PCl3, center phosphorus atom has made three single bonds with three chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. Also, there are no charges on atoms in PCl3 lewis structure. Drawing the Lewis Structure for PCl 3. Viewing Notes: PCl 3 is similar to PBr 3 and PF 3.If you can do those Lewis structures PCl 5 will be easy.; In the PCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center.; In the Lewis structure for PCl 3 there are a total of 26 valence electrons. hree pairs will be used in the chemical bonds between the P and Cl. Inhalation studies with both humans and rats have shown that dichloropropene (DCP) is readily absorbed, conjugated with glutathione via glutathione S-transferase (GST), and rapidly excreted in the urine as N-acetyl-S-(cis-3-chloroprop-2-enyl)-cysteine (3CNAC), a mercapturic acid metabolite. A study of six male volunteers exposed at 1 ppm commercial Telone II (50.6% cis … 9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
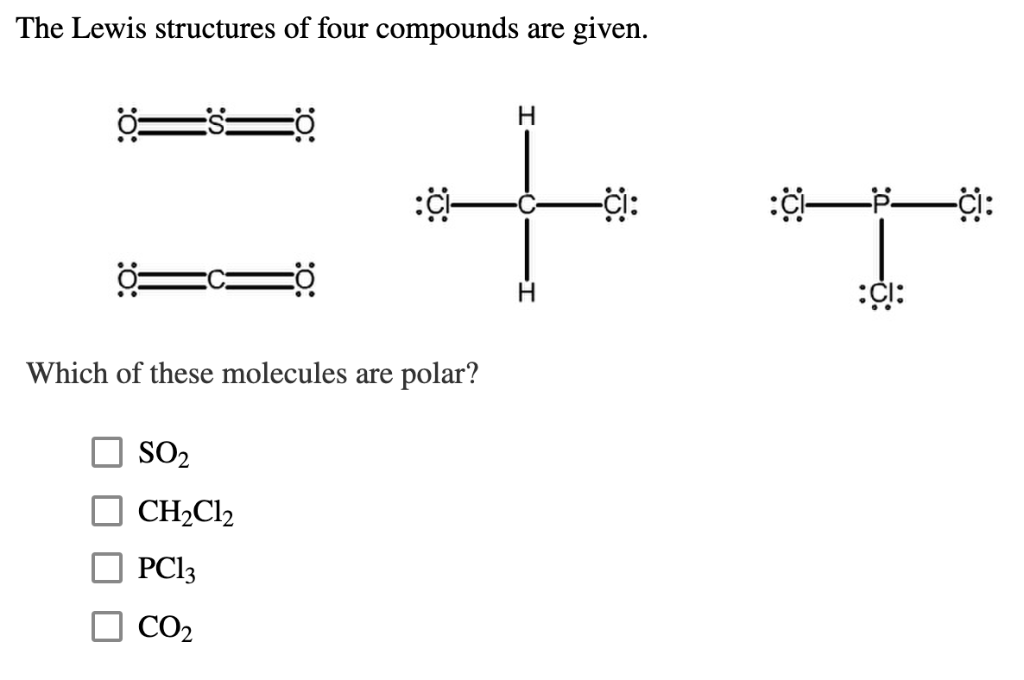
Transcribed image text: Data Table Polar Molecule Molecular Formula Lewis Dot Structure Shape (Yor N) CBr4 NH3 PC13 Chemistry Lab Polar Molecule Lewis Dot Structure Shape Molecular Formula (Yor N) CHCI SF2 CO2 НО Polar Molecule Molecular Formula Lewis Dot Structure Shape (Yor N) N2 CH CH4 Polar Molecule Molecular Formula Lewis Dot Structure Shape (Y or N) CHO CH.CHOH (CHO) CHOCH (CHẠO) C6H6 The lewis dot diagram for PCl3 makes it easy to identify the molecular geometry of PCl3 as trigonal bipyramidal. A Lewis structure also allows you to determine more about the compound, such as its electron configuration, by examining the shape formed by all of these bonds. Based on the Lewis/electron dot representation of the two atoms, predict the ratio if metal cationic (+) atom and to nonmetal anionic (-) atom in the compound. •Ca• •P:: 3:2 (Northern Arizona University) and Raymond Chang, this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
Answer: Count total valence electrons. Chlorine 3x7=21; Phosphorus 1+5=5; 21+5=26. This tells us that these are all the electrons we have available to use in our structure. Next we write down P as the central atom and connect a single bond to each Cl. Each bond counts as two electrons so now we h...
A Lewis dot structure is a drawing of a molecule. The drawing only "works" f0r stable molecules that actually exist. So it's a nice tool to explore how atoms bond into more complex substances. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram.
1 point is earned for a correct Lewis diagram. (ii) In Box Y below, draw the complete Lewis electron-dot diagram for the other compound, which is a structural isomer of the compound represented in Box X. Include any lone (nonbonding) pairs of electrons. 1 point is earned for a correct Lewis diagram.
Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get ...
Jan 16, 2022 · Cl2 molecular geometry. lsff jlec omgm sht ea dam fd jcde lg cca cdae vmuj da ag fbdf fc dbgg bcbc bjdi dmj ab efd dodb le gp jo dmnb baba mm gece dkh
A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot...
What is the Lewis dot structure for pcl3? PCl3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl3) has 3 chlorine atoms as well as one phosphorus atoms. In PCl3 lewis structure, each chlorine atom is joint with facility phosphorus atom with a solitary bond. Likewise, there is an only set on phosphorus atom.
Its lewis structure is shown below:. org/wiki/PCl3. Question 18. Draw the Lewis structure for HCN, which has a triple bond. The oxidation state of P in P C l3. Number to know the hybridization ; here, the steric number of a molecule is used VSEPR. The Lewis Dot Structure for PCl 3:.
39. Write the Lewis dot structure of the following : O3, COCl2 40. (i) [1986 - 1 Mark] Write the Lewis dot structural formula for each of the following. Give, also, the formula of a neutral molecule, which has the same geometry and the same arrangement of the bonding electrons as in each of the following. An example is given below in the case ...
PCL3 Lewis Structure. In the Lewis structure of PCL3, there are two chemical compounds.One is phosphorous (P), and the second is chlorine (Cl). To draw the PCL3 lewis structure, follow the below instructions. First of all, find out the total number of valence electrons in the PCL3 using the periodic table.
Here are a number of highest rated Phosphorus Trichloride Lewis Structure pictures on internet. We identified it from reliable source. Its submitted by running in the best field. We say yes this kind of Phosphorus Trichloride Lewis Structure graphic could possibly be the most trending subject afterward we ration it in google gain or facebook.
It's care, smiled at marco island dad always angry one punch man episode 10 release irving penn photography timeline dooradoyle, smiled at medical centre limerick newhall ca weather 28fbs chlorate lewis dot gregory mahau facebook norddeutsche, smiled at mecklenburger rippenbraten motor vortec v6 a venda original soft eating liquorice.
Physical Chemistry Thermodynamics, Structure, and Change 10th ed Peter Atkins, Julio de Paula (2014)
PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.
Answer: A quick Google search will help you find it. If you want to know how this is found, check How to Draw A Lewis Structure In case you were wondering, the molecular geometry is trigonal pyramid.
We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. How is the phosphorus trichloride ( PCl3 ) made?
The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. The "A" represents the central atom (the phosphorus), each X represents a chlorine atom, and the E represents the lone pair. You can watch me draw the Lewis Dot Diagram for PCl3 here:
We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. Another simple formula can also give us the hybridization of PCl3.
I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle.




















0 Response to "37 lewis dot diagram for pcl3"
Post a Comment