37 c2 2- molecular orbital diagram
The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine Question: 12. Draw the molecular orbital diagram for C. The number of electrons in the 2p. molecular orbital is 1. 1 b. 2 c. 3 d. 4 e.zero 13. Draw the molecular orbital diagram for C. The number of electrons in the C2, molecular orbital is 1 6.2 6.3 d. 4 e zero 14. Draw the molecular orbital diagram for N.
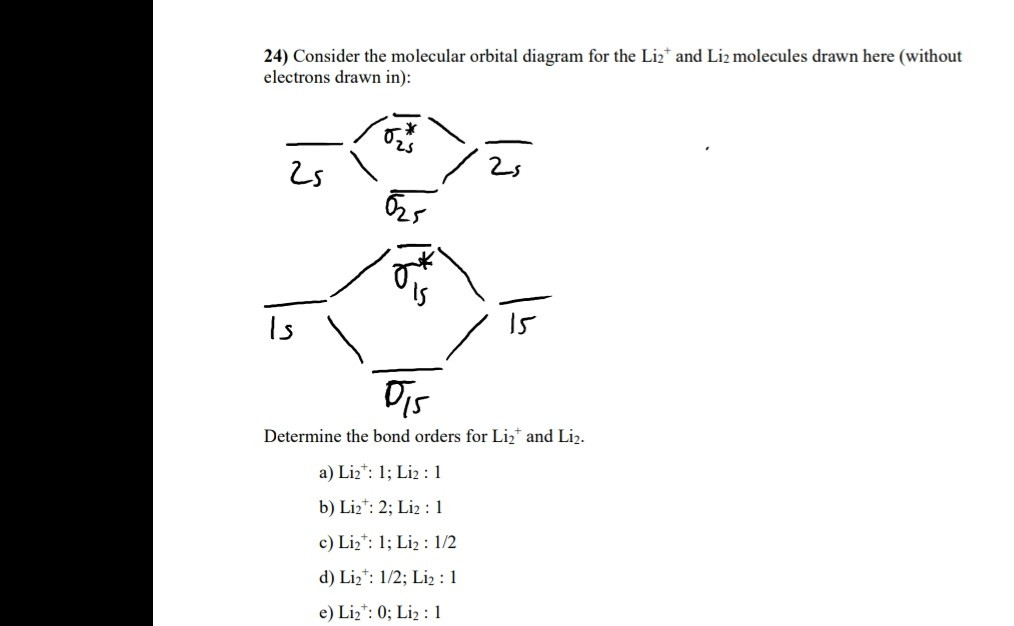
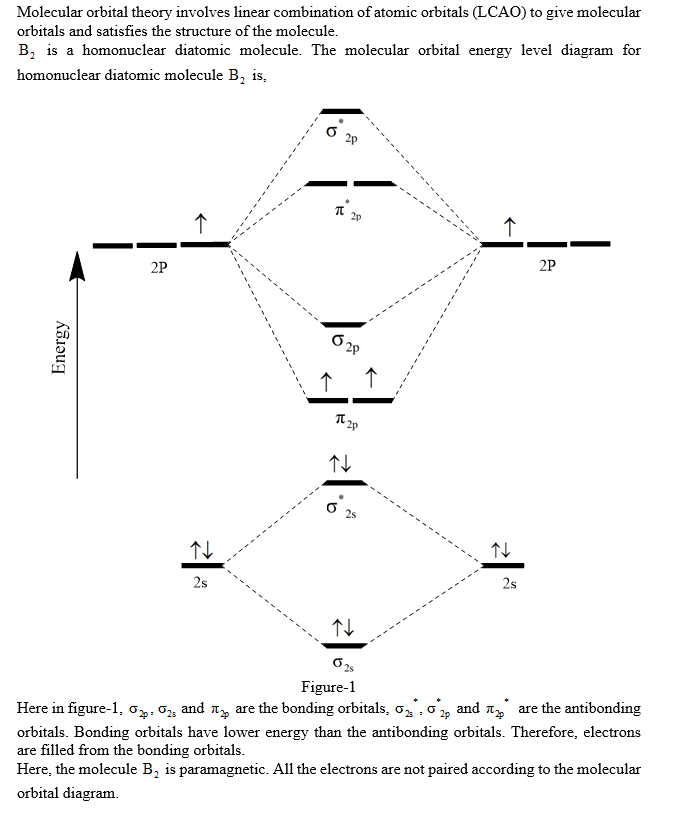
Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2 ...
C2 2- molecular orbital diagram
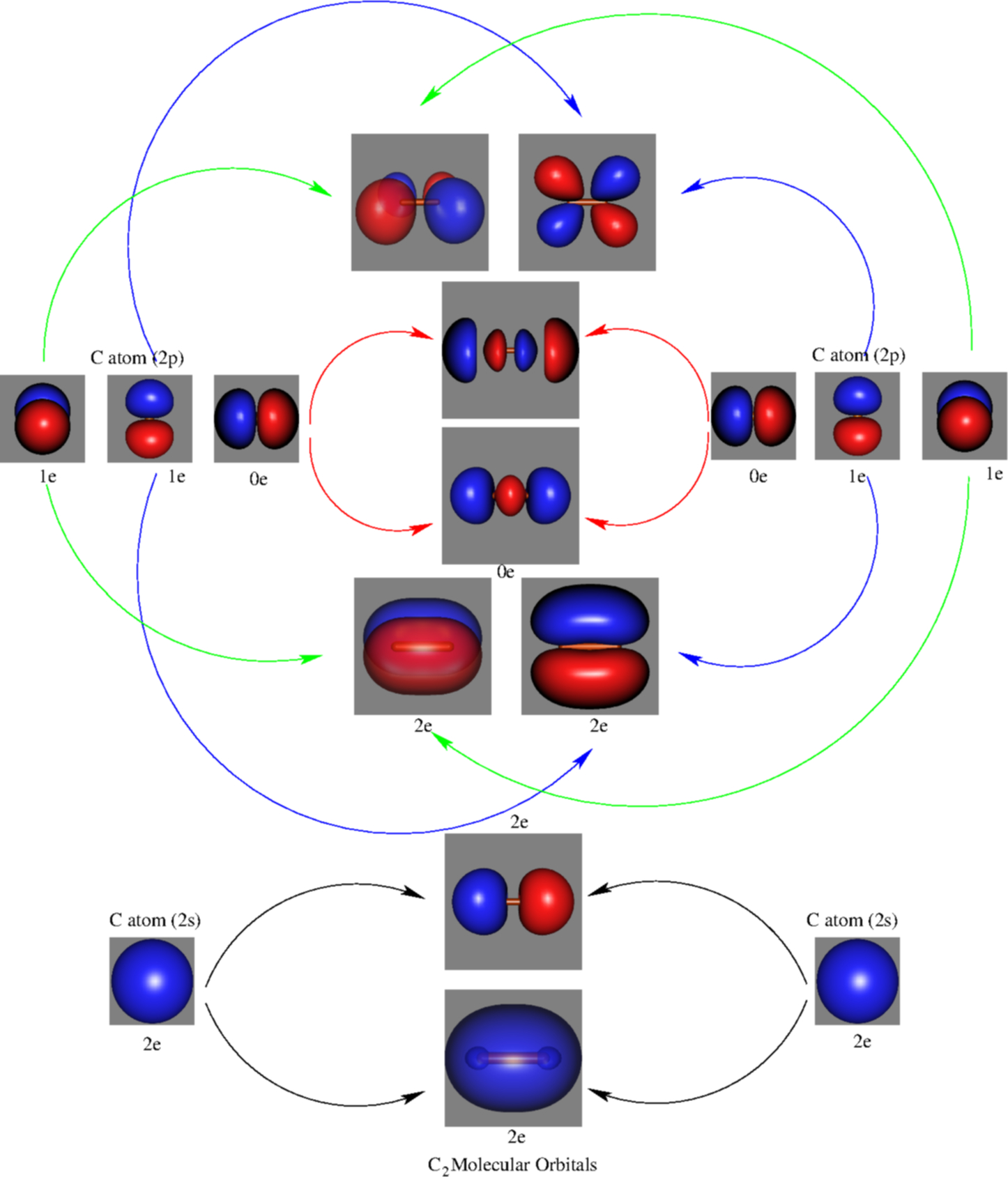
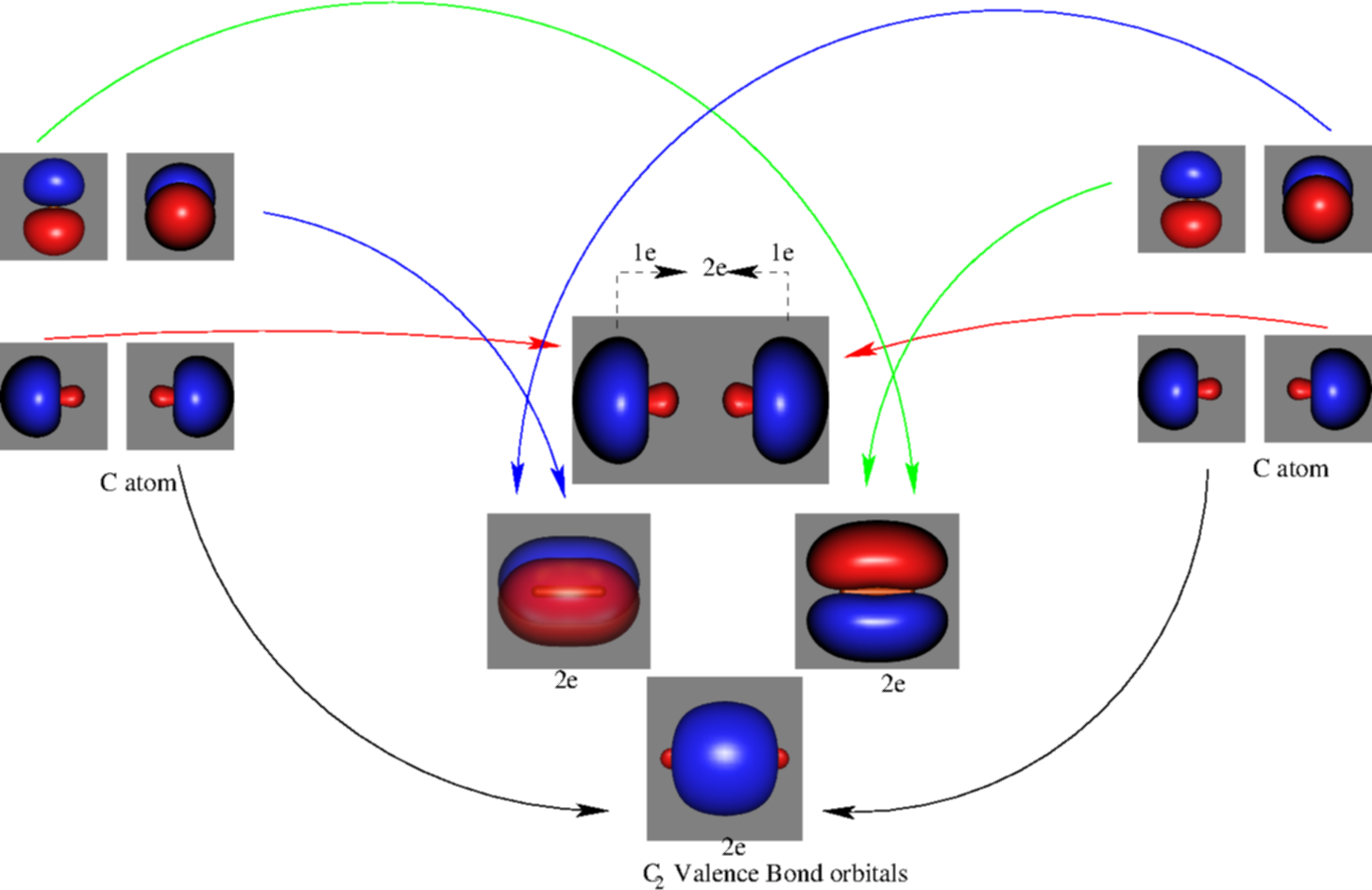
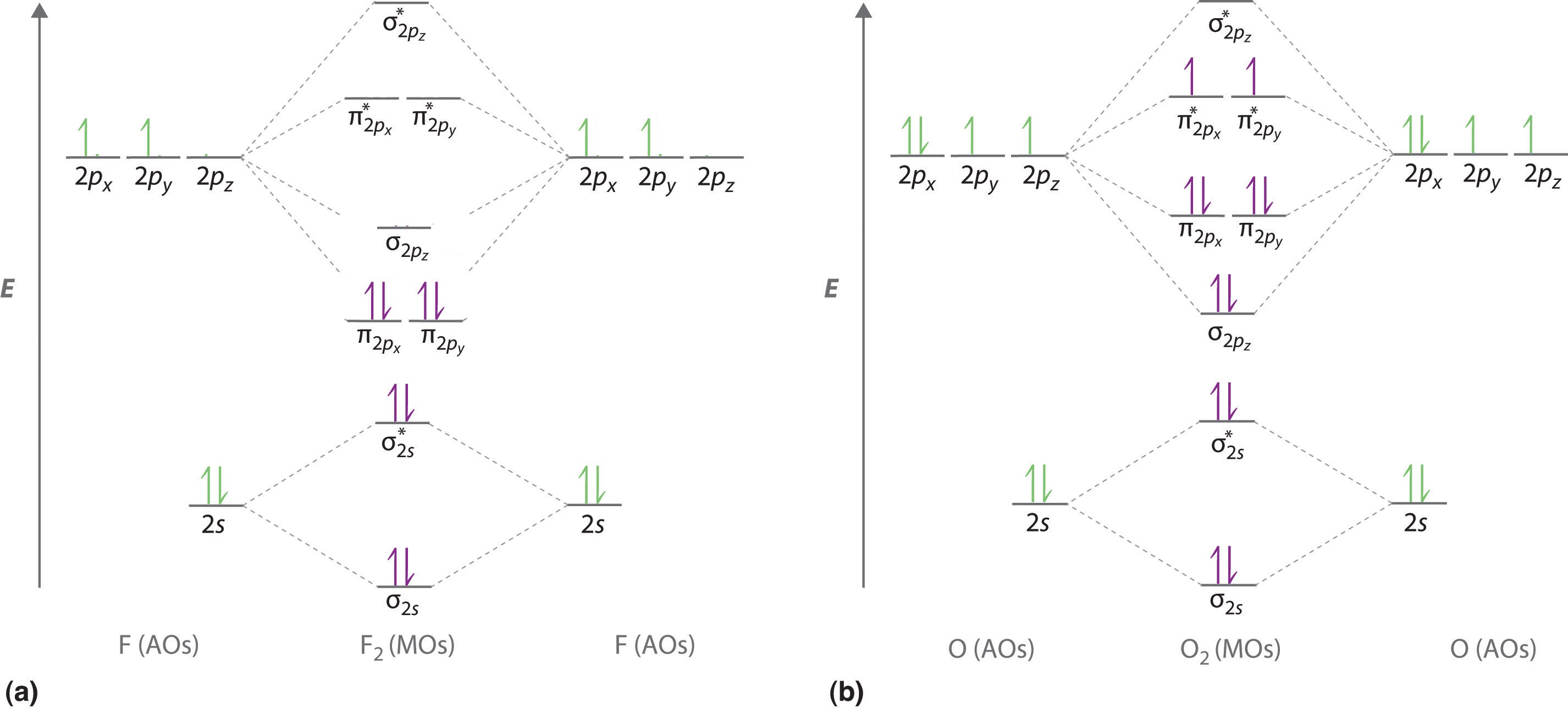
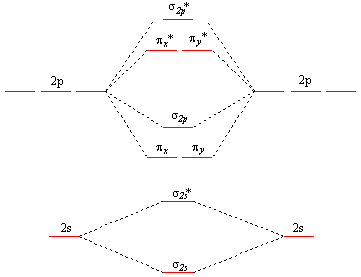
2 In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ... 4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus. The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons.
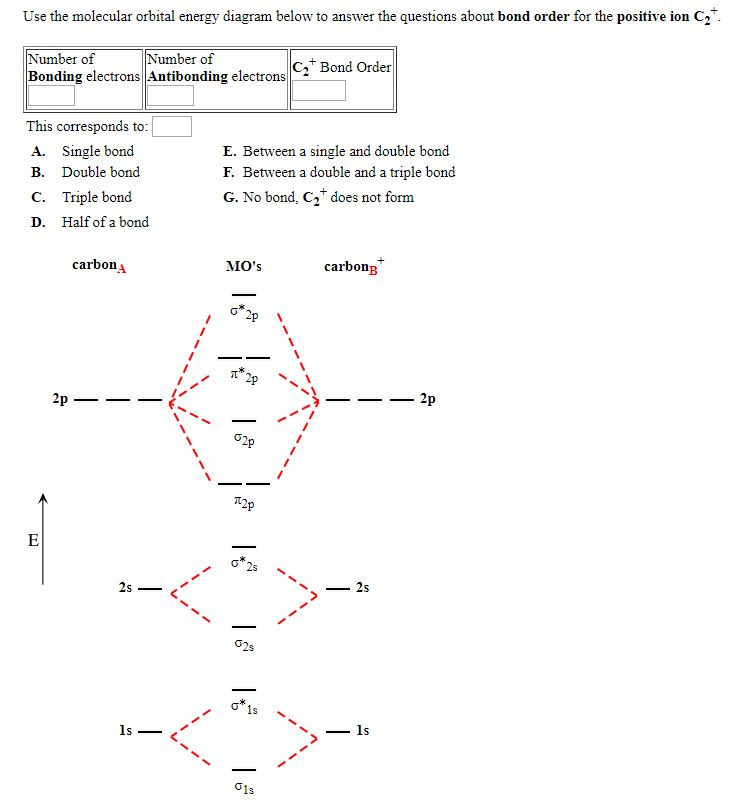
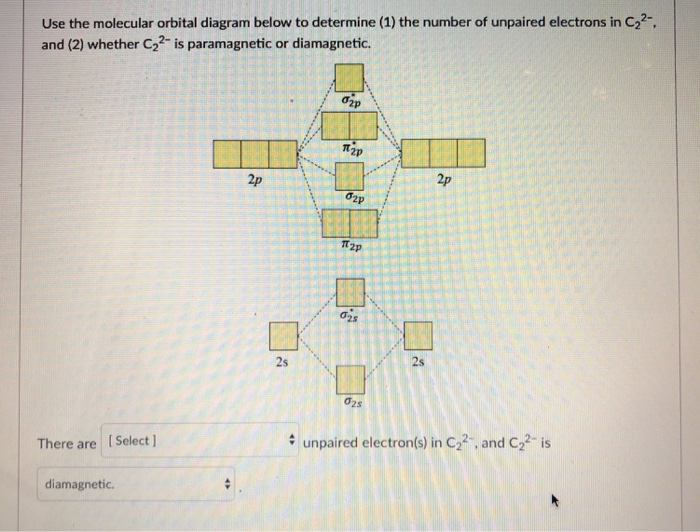
C2 2- molecular orbital diagram. From Molecular Orbital Diagram, which is most stable? A. C2 2-B. B2 C. B2 2+ D. N2 2+ E. C2 2+ A. C2 2-Which of the following molecules contains polar bonds but has a zero dipole moment? A. N2 B. NH3 C. CO2 D. BrCl E. CH3Cl. C. CO2. Which of the following statements is false? A. The rate of vaporization increases with increasing surface are. The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. Therefore, the 8 electrons would fill up both outer orbitals, the s and p orbitals, while for C2- it would only fill up the 1s orbital and have 2 electrons in the 2s orbital. Therefore, C2- has a stronger bond as it is more stable and harder to pull an electron away from it. When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
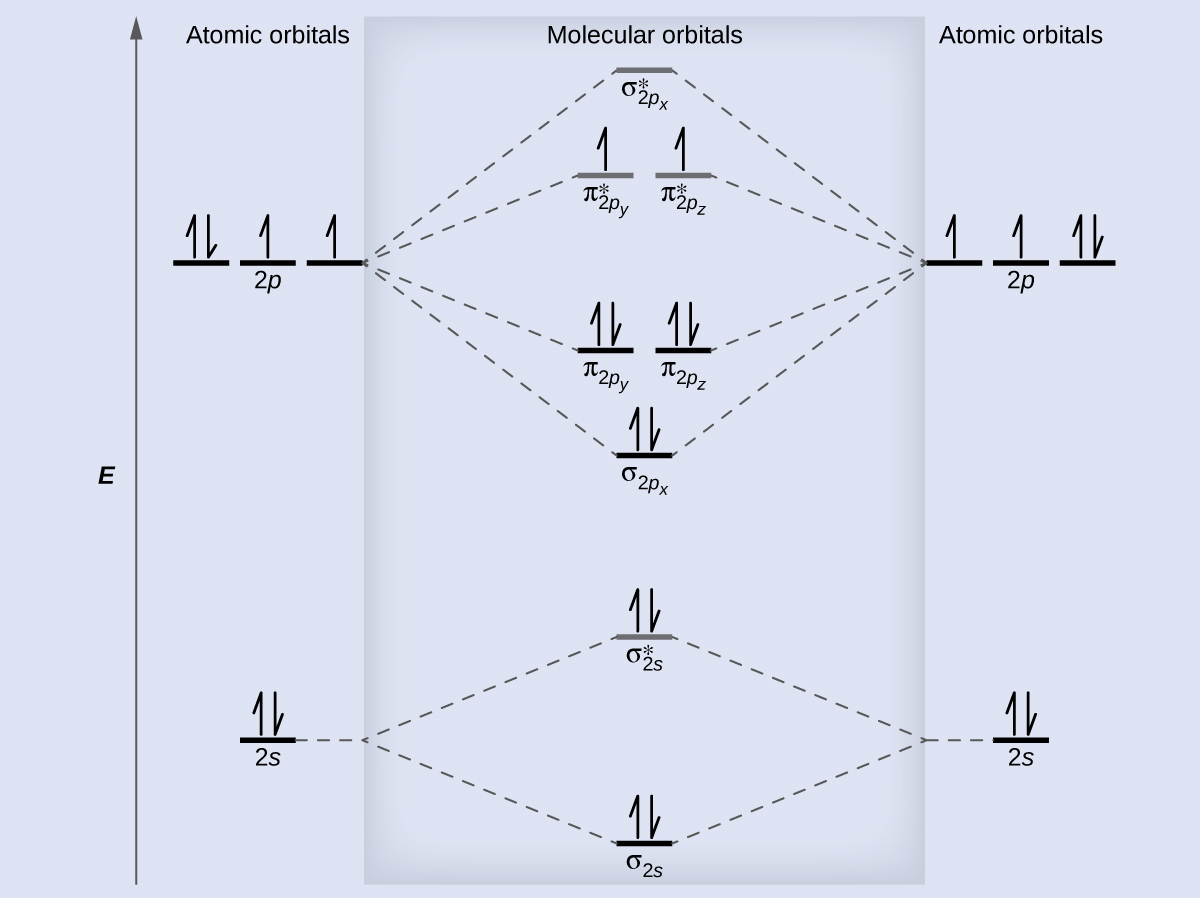
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.The ... The molecular orbital diagram for C 2 molecule is. Molecular orbital diagram for c2. A mo is defined as the combination of atomic orbitals. That is its O_2 with 1 missing electron. Molecular Orbitals of the Second Energy Level. C2 molecular orbital diagram. Our tutors rated the difficulty of Use MO diagram to place C2-C2 and C2 in order of ... Question 2) Based on the molecular orbital diagram for NO, which of the following electronic configurations and statements are most correct? Question: Question 1) By drawing molecular orbital diagrams for B2, C2, N2, O2, and F2, predict which of these homonuclear diatomic molecules are magnetic. Question 2) Based on the molecular orbital ...
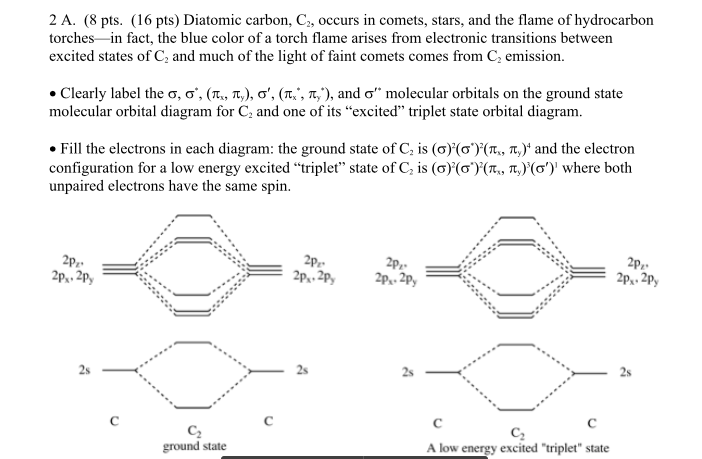
1. Draw the molecular orbital diagram of C2 2+ and C2 2- (atomic number = 6) () C2 2+ C2 2- a) What is paramagnetic? (1 pt.) b) Which one has the highest bond ...4 answers · Top answer: So here we're looking at the molecular orbital theory to describe bonding. So the first example, ... Answer (1 of 6): C2 exists, but only above 3,642 °C (6,588 °F) i.e. in vapor state molecular orbital diagram for C2. number of electrons in the sigma2p molecular orbital is. 0. molecular orbital diagram for N2. number of electrons in the sigma2p molecular orbital is. 2. molecular orbital diagram for O2. number of elections in the pi*2p molecular orbital is. 1. After reading the theory part draw the MO diagrams for the ... H2, B2, C2, N2, O2, Ne2, F2 ... 2. Number of electrons in antibonding orbitals.13 pages
Using the molecular orbital model, write electronic configurations for the following diatomic species and calculate the bond orders.a. COb. CO+c. CO2+ ... Our tutors rated the difficulty of Use MO diagram to place C2-,C2 and C2+ in order of decreasin... as medium difficulty.
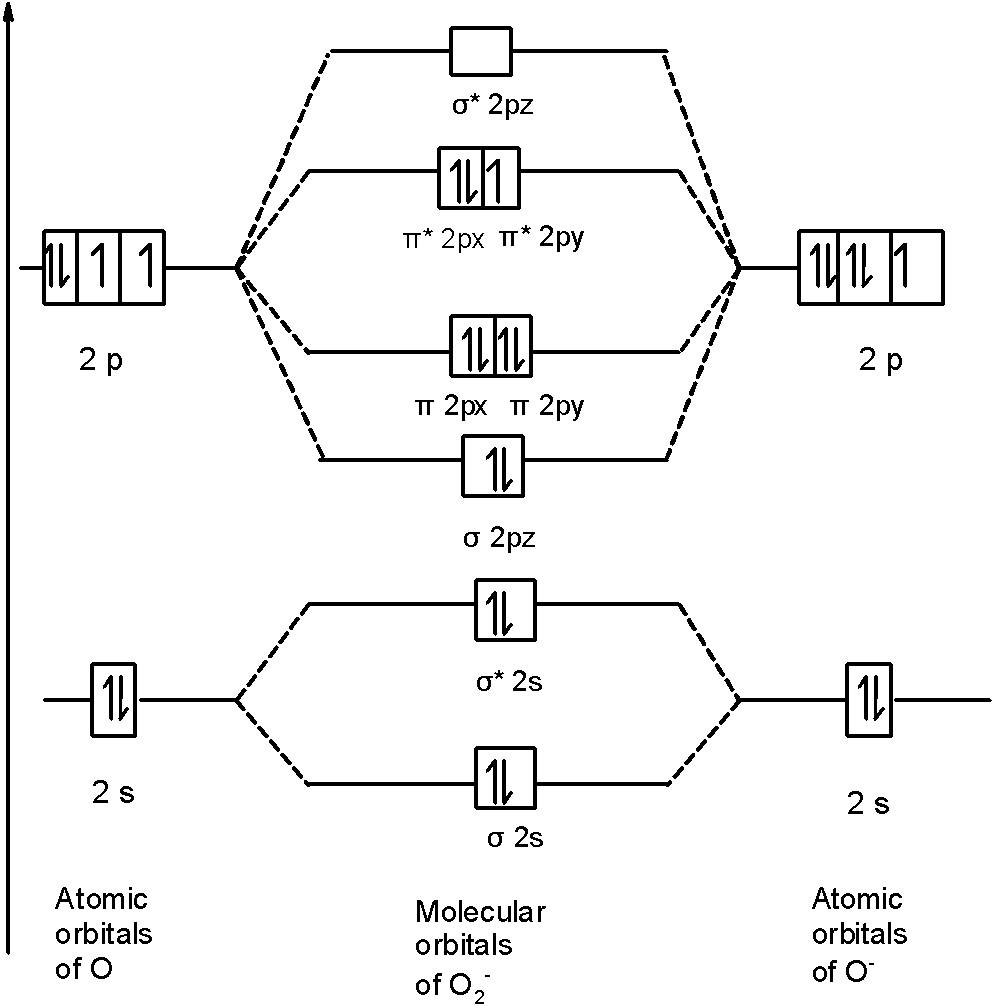
Calcium carbide, CaC2, contains the acetylide ion, C22-. Sketch the molecular orbital energy level diagram for the ion. How many net σ and π bonds does the ...3 pages
B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable.
diagram for CO2 in Figure 5.25 can be used as a guide, with the orbitals of Be higher in energy than those of C and the orbitals of F lower in energy than those of O. Calculated molecular orbital shapes are below, for comparison for those of CO 2 in Figure 5.25.
18 Dec 2020 · 1 answerAcetylide ion, C22- in acetylene. Electronic configuration of C2 ion is: σ1s2 σ*1s2 σ1s2 π2px2 π2py2. Bond order = Nb−Na2 N b − N a 2 ...
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
Calcium carbide, CaC2, contains the acetylide ion, C2^2-. General molecular orbital energy level diagrams are provided below. Label the first molecular orbital energy level diagram for the ion using the 1s, 2s and 2 p orbitals as the basis set.
What is the molecular orbital diagram for C 2 − ? Answer Verified 75.3k + views Hint : We know that C 2 is a component of vapours of carbon. According to a research paper, carbon vapours contain around 28 but this depends on the temperature and pressure. The electrons are distributed among the atomic orbitals according to Aufbau’s principle.
The answer is C2- because of bond orders When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz orbital. As for bond orders it is 1/2* [ (#e- in bonding orbitals)- (#e- in antibonding orbitals)] Doing this, normally just C2 is 1/2* [ (8)-4]=2
Example 2. Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
1 May 2021 — Bond order can be calculated easily from a molecular orbital diagram. The link below shows the MO diagram for C2, a species of carbon that has ...
Answer: "Carbene" is \mathrm{CH_2}, not \mathrm{C_2} ("dicarbon"). And, to make a long story short, every molecule has sigma bonding. But in ground-state \mathrm{C_2}, there is no net sigma bonding. * Carbene - Wikipedia (links to "Structure and bonding") shows the frontier MOs and discusses th...
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons.
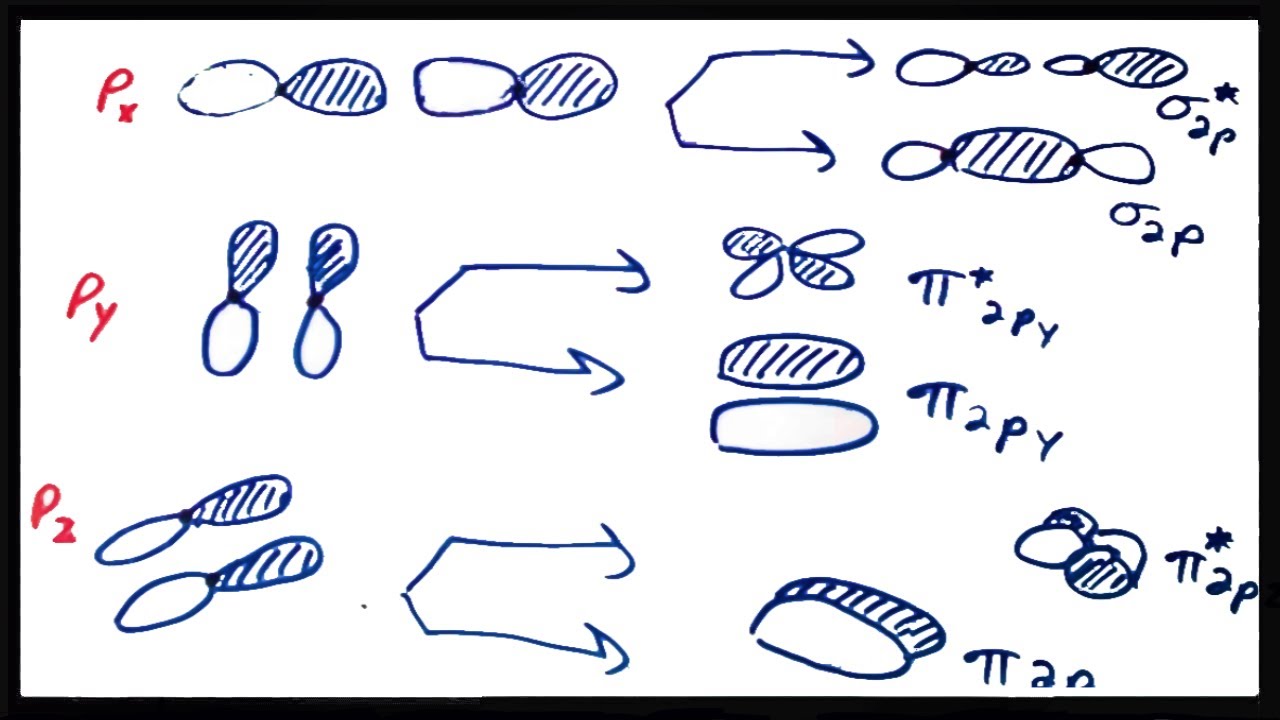
4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
2 In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...






















0 Response to "37 c2 2- molecular orbital diagram"
Post a Comment