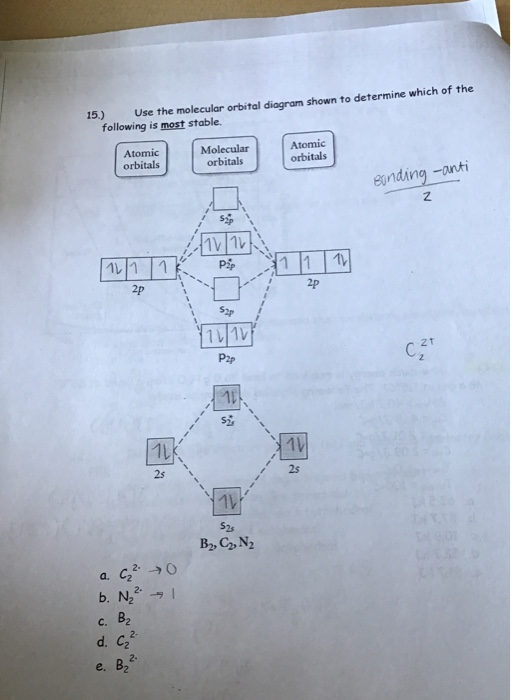
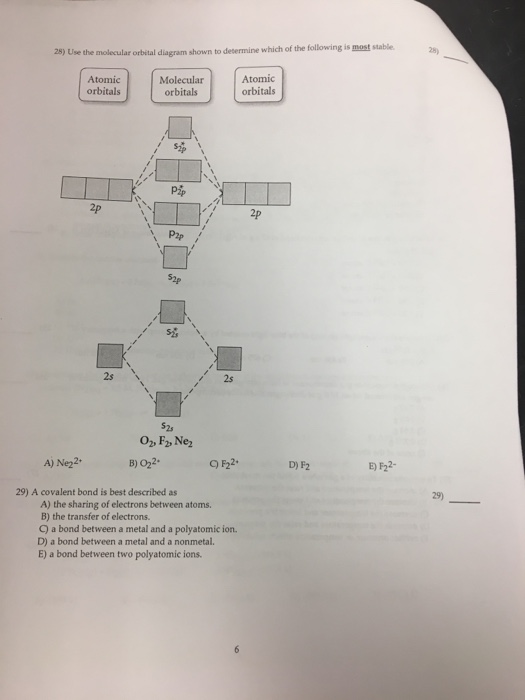
37 use the molecular orbital diagram shown to determine which of the following is most stable
Use the molecular orbital diagram shown to determine which of the following is most stable. A. F22+ B. Ne22+ C. F22- D. O22+ E. F2 - 3580227 Problem: Use the molecular orbital diagram shown to determine which of the following is MOST stable. In molecular orbital names, s = sigma and p = pi.a.1 answer · Top answer: O22+
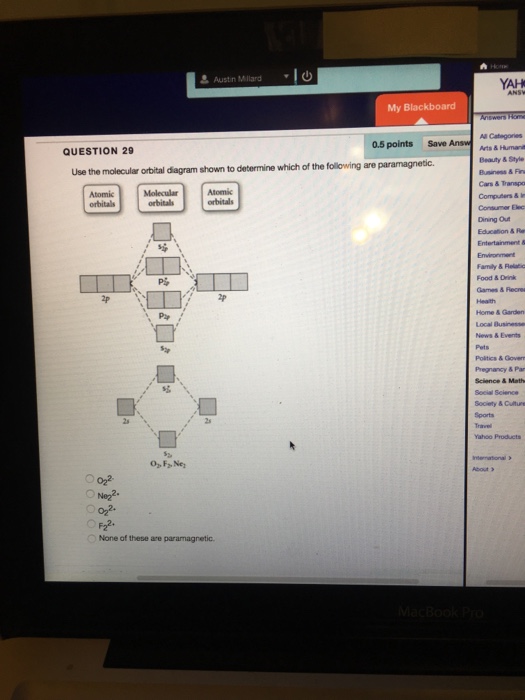
Use the molecular orbital diagram shown to determine which of the following is most stable A) Ne2^2⁺ B) F2^2⁺ C) F2^2⁻ D) F2 E) O2^2⁺ E). O2^2⁺ Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A) O2 2⁺ ...
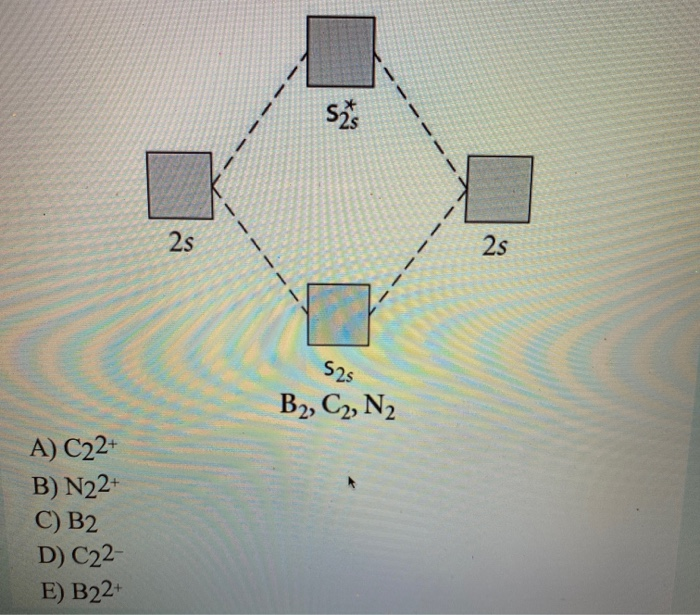
Use the molecular orbital diagram shown to determine which of the following is most stable
​ ## [RADIANCE] Greetings fellas. I loathe myself for putting this here right near the deadline. At least i hope i will entertain you at least a little. Radiance: This ability works as manipulation of interconnected subatomic properties by proxy, mainly by manipulating patterns of the strong interaction. In fact, it operates by changing quantum states of quarks by preventing and manipulating their combination into various hadron types, preventing the formation of hadrons, such as... Transcribed image text: Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order Atomic orbitals ... Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Use the molecular orbital diagram shown to determine which of the following is most stable. Use the molecular orbital diagram shown to determine which of the following is most stable. Use the molecular orbital diagram shown to determine ... Subjects. Science. Chemistry Video Lessons Exam Reviews ACS Video Solutions Solutions Library. Organic Chemistry Video Lessons Exam Reviews ACS Video Solutions Solutions Library. Physics Video Lessons Exam Reviews Solutions Library. Biology Video Lessons Exam Reviews Solutions Library. E. For. The valence electrons = 14; BO = 0.5* (8-6) = 1. The bond order is commonly used to signify the bond stability. Higher bond order indicates more stability and vice versa. Thus, is the most stable. answered: emily4984. SHOW ANSWER. The one that is most stable is F2 or E. Eg: H + H two 1s orbitals mix to form sigma and sigma*. Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding orbitals, the compound is stable. Eg: He + He; same mixing as above. Four electrons, two in the sigma, two in the sigma*. Since there are as many bonding electrons as as antibonding, there is ...
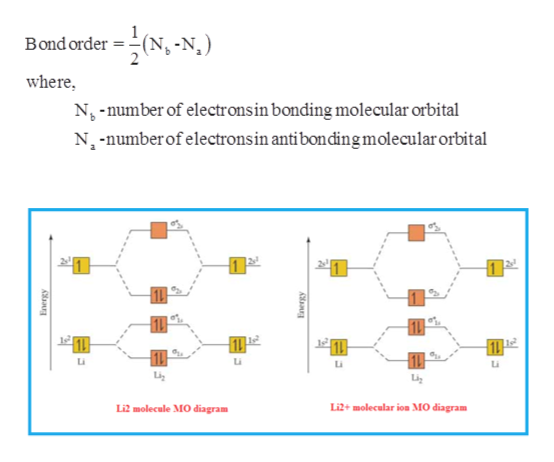
Use the molecular orbital diagram shown to determine which of the following is most stable. ... For All Answers. For All Questions And Answers. Menu Home; Posted on October 23, 2021 by sarah yalton. Use the molecular orbital diagram shown to determine which of the following is most stable. Use a molecular orbital energy-level diagrams to predict the bond order and stability of the \(\ce{He_2^{2+}}\) ion. Given: chemical species. Asked for: molecular orbital energy-level diagram, bond order, and stability. Strategy: Combine the two He valence atomic orbitals to produce bonding and antibonding molecular orbitals. Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. The fourth principle states that stable molecular orbitals are easiest to form when constructed out of atomic orbitals of similar energies. This means that 1s orbitals should combine with 1s orbitals and 2p orbitals should combine with 2p orbitals etc. to form the most stable molecular orbitals.
Question: Use the molecular orbital diagram shown to determine which of the following is MOST stable. This problem has been solved! See the answer ... Use molecular orbital diagram shown to determine which is most stable a o22 bf2 c f22 d f22 e ne22 a. A asdfasdf b asdfasdf c asdf d f2 2 e none of the above are paramagnetic. Label each and each and every molecular orbital with its call sigma pi and position the accessible electrons interior the perfect atomic orbitals and molecular orbitals. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:Ne22+O22-F22+O22+None of the above are paramagnetic. Using the appropriate molecules orbital diagram, determine the most stable form of the following species: F2,... Use the molecular orbital diagram shown to determine which of the following is most stable. Atomic orbitals Molecular orbitals Atomic orbitals 吣 2p 2p P2p ...
31) Use the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2⁺ B) N2^2⁺ C) B2; D) C2^2⁻ E) B2^2⁺ 32) How many moles of oxygen are formed when 58.6 g of KNO3 decomposes according to the following reaction? The molar mass of KNO3 is 101.11 g/mol. 4 KNO3(s) → 2 K2O(s) + 2 N2(g) + 5 O2(g)
🔴 Answer: 1 🔴 on a question Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2 - the answers to answer-helper.com
Use the molecular orbital diagram shown to determine which of the following is most stable. a. f22+ b. ne22+ c. f22- d. o22+ e. f2
## [RADIANCE] Greetings fellas. I loathe myself for putting this here right near the deadline. At least i hope i will entertain you at least a little. Radiance:This ability works as manipulation of interconnected subatomic properties by proxy, mainly by manipulating patterns of the strong interaction. In fact, it operates by changing quantum states of quarks by preventing and manipulating their combination into various hadron types, preventing the formation of hadrons, such as protons and neut...
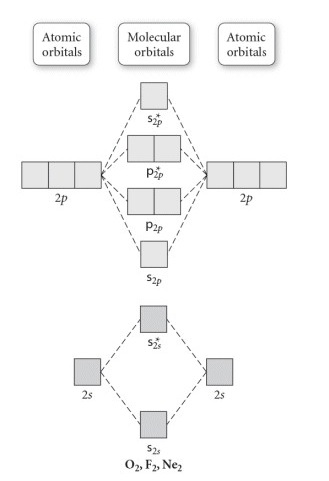
Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order. Atomic orbitals Molecular orbitals Atomic orbitals O, F, Ne Ne22 F₂2. F2 . 022- • F22. Question: Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order.
Use the molecular orbital diagram shown to determine which of the following is most stable. Use the molecular orbital diagram shown to determine which of the following is most stable. D c 2 2. Start studying exam 3. Inorganic Chemistry Why Do Compounds Like Sf6 And Sf4 Exist But What Is The Molecular Orbital Diagram For O2 And O2 Ions Quora
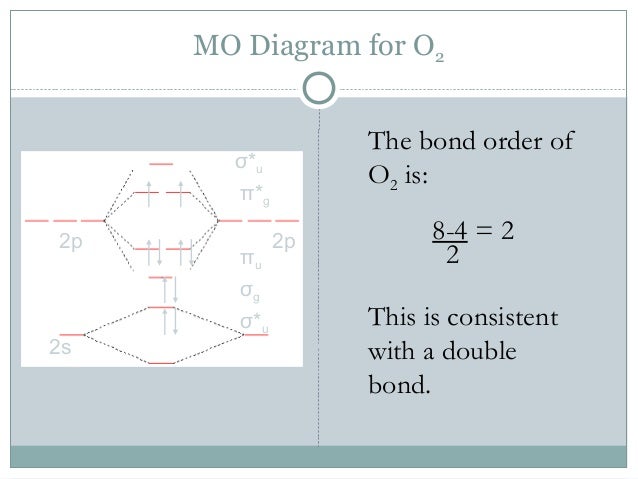
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Transcribed image text: Use the molecular orbital diagram shown to determine which of the following is most stable. Please note that the number of valence ...
Chemistry questions and answers. Part A Use the molecular orbital diagram shown to determine which of the following is most stable Atomic orbitals Molecular orbitals Atomic orbitals TE 2p 2p 22 Tap 25 BCN NA? 82 MacBoo Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals ...
Use the molecular orbital diagram shown to determine which of the following is most stable. The three 3 component mixture shown below was spotted to a tlc plate and developed using the solvent system listed. 1 draw the molecular orbital diagrams to determine which of the following is most stable.
a. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B_2^2+, B2, C_2^2-, B_2^2- and N_2^2+ b. Draw the Lewis structures and molecular orbital diagrams ...
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b. Ne22+c. F22-d. O22+e. F2. FREE Expert Solution. Recall that the bond order t ells us the strength and length of a bond: a higher bond order means the bond is stronger and shorter.
3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2; D) C2^2-E) B2^2+ 5) Which statement regarding stable heteronuclear diatomic ...
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Use the molecular orbital diagram shown to determine which of the following is most stable. Identify the number of bonding pairs and lone pairs of electrons in water. When two atomic orbitals come together to form two molecular orbitals one molecular ...
Draw the molecular orbital diagram shown to determine which of the following is most stable. F22+ F2 Ne22+ O22+ F22-. This problem has been solved! See the ...
Use molecular orbital diagram shown to determine which is most stable a) O22-b)F2 c) F22+ d) F22-e) Ne22-a. Use the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ...
Draw the Molecular orbital Diagram Shown to Determine which Of the Following is Most Stable. use the molecular orbital diagram shown to determine which use the molecular orbital diagram shown to determine which of the following is most stable 38 a b2 b c22 use the molecular orbital question draw the molecular orbital diagram shown to answer to draw the molecular orbital diagram shown to ...
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
Transcribed image text: Use the molecular orbital diagram shown to determine which of the following is most stable based on their bond order Atomic orbitals ...
​ ## [RADIANCE] Greetings fellas. I loathe myself for putting this here right near the deadline. At least i hope i will entertain you at least a little. Radiance: This ability works as manipulation of interconnected subatomic properties by proxy, mainly by manipulating patterns of the strong interaction. In fact, it operates by changing quantum states of quarks by preventing and manipulating their combination into various hadron types, preventing the formation of hadrons, such as...

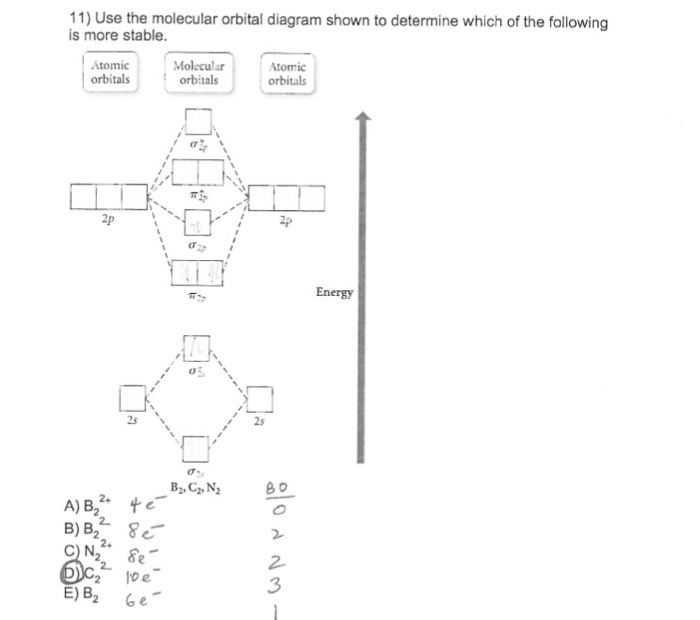


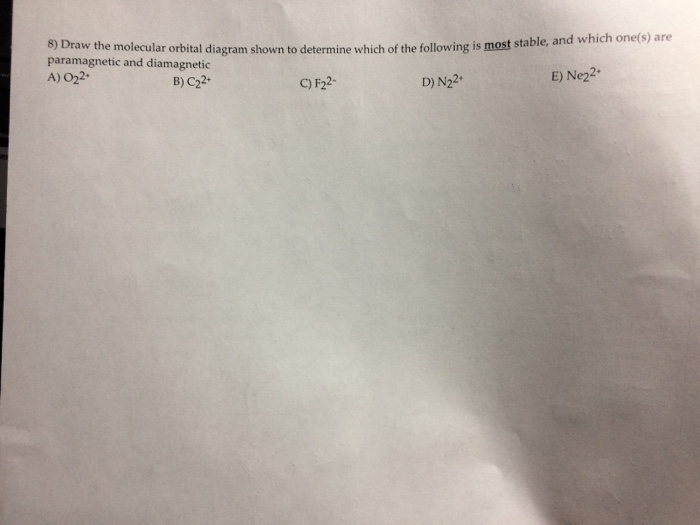



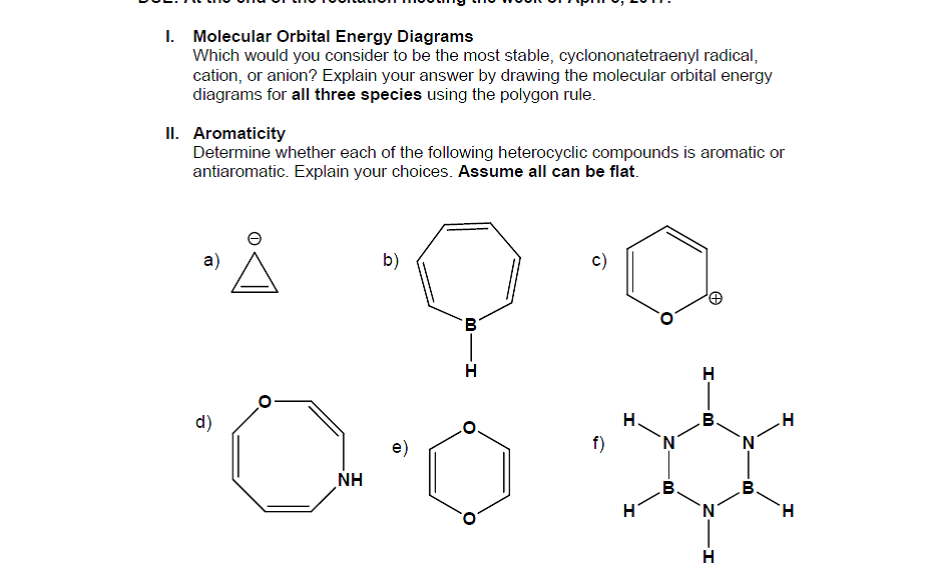



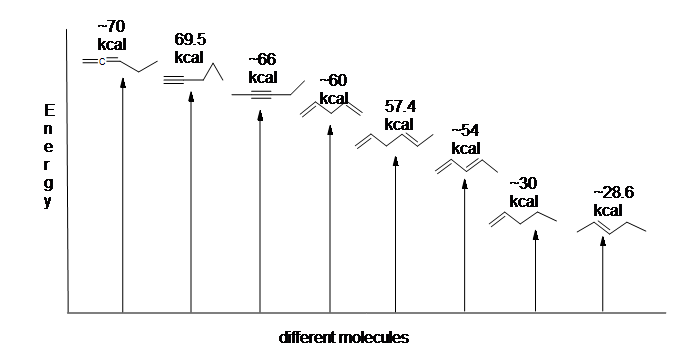
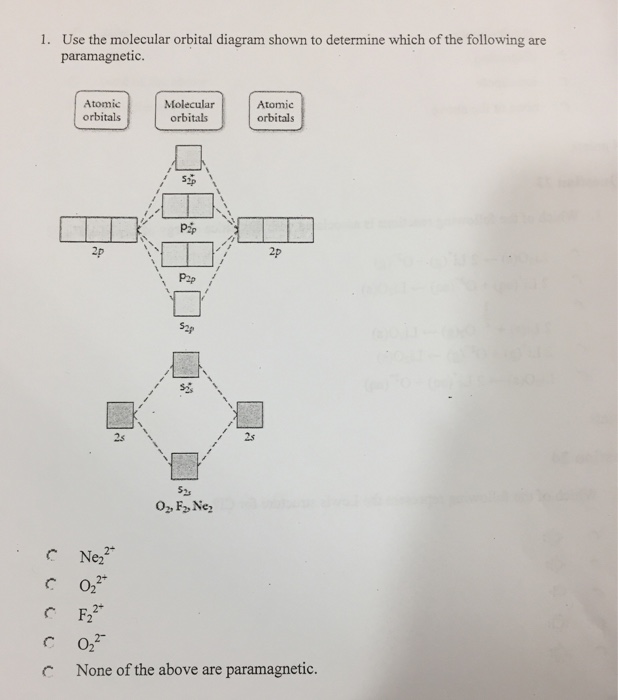




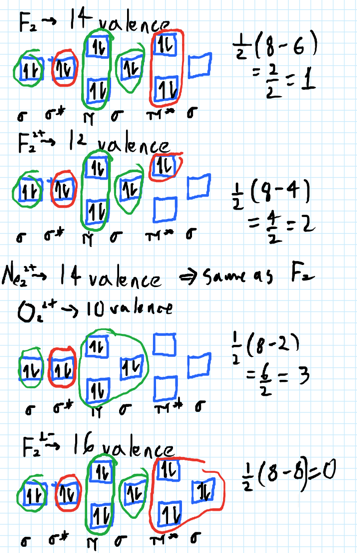

0 Response to "37 use the molecular orbital diagram shown to determine which of the following is most stable"
Post a Comment