35 lewis dot diagram n2
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen. Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
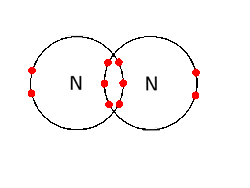
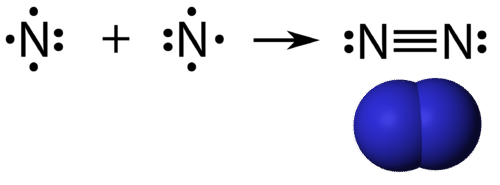
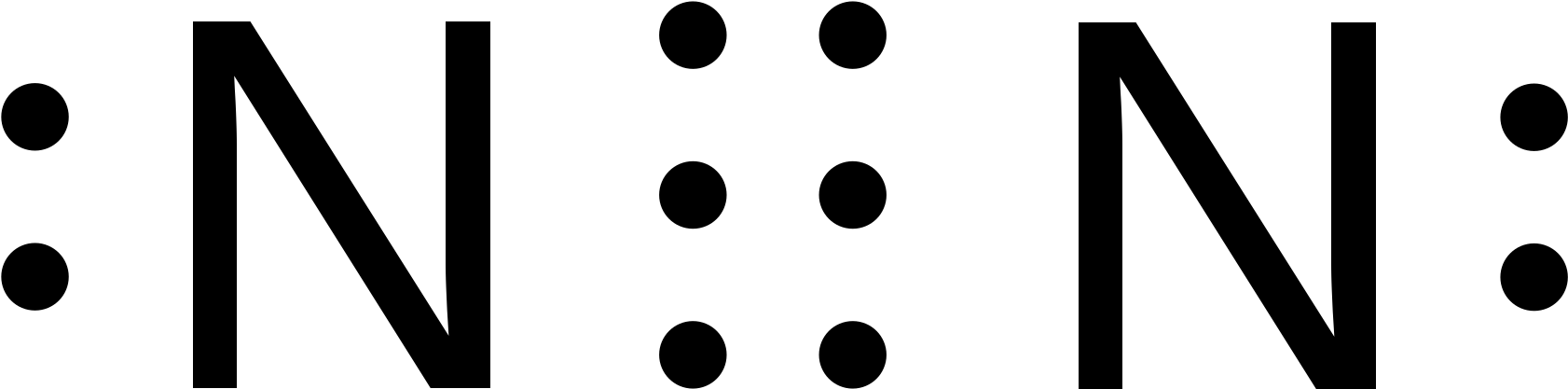
N2 dot structure would comprise of two atoms of Nitrogen(N) atoms. There is a triple bond between both nitrogen atoms. Each N is surrounded by two dots which are called lone pairs of electrons. It is a diatomic nonpolar molecule with bond angles of 180 degrees. 6. Explain o2 lewis structure in simplest form. Two oxygen atoms are joined by a ...

Lewis dot diagram n2
The dot structure of Na+1 is Na+1 . The dot structure of O-2 is O-2. Note that Na is in group 1 and should lose 1 electron while O is in group 6 and should gain 2 electrons. ionic compounds Make certain it's ionic: one atom must be from groups 1-3, the other from groups 4-7 (including H). Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell.As per the molecule N2, it has two atoms of Nitrogen. Lewis Dot Structure for Compounds. Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows ...
Lewis dot diagram n2. The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. One $\ce{N2}$ molecule formed: What you have drawn here is (as I have understood) Lewis dot diagram of the $\ce{N2}$ molecule. The correct way of drawing the molecule is as in 1) Two separate N atoms which have not yet formed any bond: With the concept of Lewis dot structure formula the Nitrogen atoms are represented as in 2) NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3. Lewis dot structure of n2 molecule Let's take a look at the Lewis of and N2 structure. Atomic nitrogen has 5 valence electrons and 4 orbital valence (2s, 2 px, 2py and 2pcs). In the Lewis structure there is a triple link between nitrogen atoms and a pair of non-binding electrons on each. This is consistent with the physical properties of N2.
What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ... A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons. A step-by-step explanation of how to draw the N2 Lewis Dot Structure (Nitrogen Gas - Diatomic Nitrogen).For the N2 structure use the periodic table to find t... The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . .
on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use . N2 Lewis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It's easiest to think in terms of […] Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas). Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth's atomsphere is N 2).It also is a good example of a molecule with a triple bond. There are 10 valence electrons available for the Lewis structure for N 2.. Video: Drawing the Lewis Structure for N 2 Lewis Dot Diagram For So4 2. Simple procedure for drawing covalent Lewis structures - Lewis dot of the sulfate ion SO, best lewis structure for so, electron bonding. Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis.
The following is the Lewis Dot Structure for N2, 2N and N2, are basically two forms of the same element. There is a little difference between the two. 2N refers to two molecules of Nitrogen atom, and N2 states that two atoms of Nitrogen are present in a single molecule. The number written at the start, refers to the number of molecules and the ...
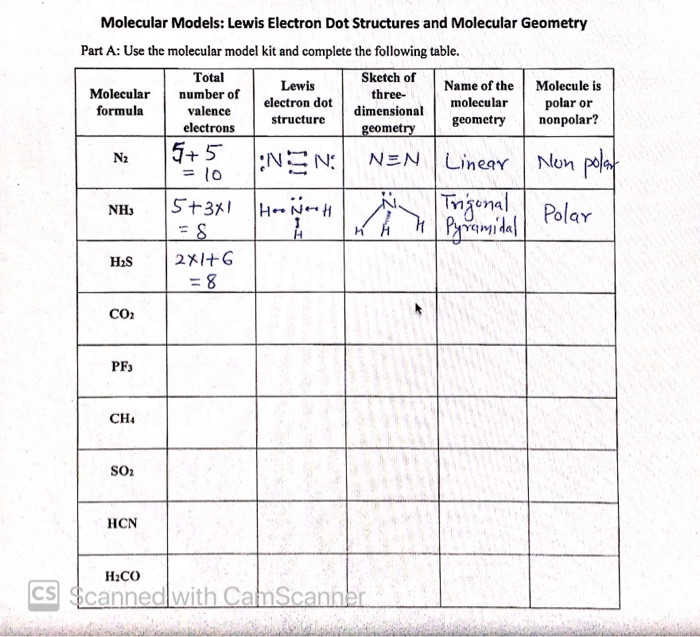
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen--it's in group 5 or 15 on the periodic table. We have two Nitrogens. Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We'll put the two Nitrogens next to each other, and then we'll put two valence electrons between them to form a chemical bond.
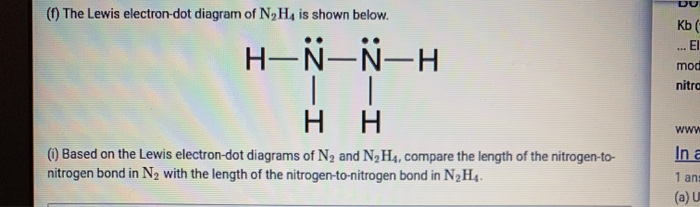
Based on the Lewis electron-dot diagrams of N2 and N2H4, N2 has a stronger nitrogen-to-nitrogen bond than N2H4.. The strength of a bond is dependent on the bond length and the bond order.The higher the bond order, the shorter and stronger the bond.Hence triple bonds are stronger than double bonds and double bonds are stronger than single bonds.. Having said that, N2H4 contains single bonds ...
Lewis dot diagram for n2. Each nitrogen atom also has a lone pair of electrons. The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible. The lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6 steps.
Answer (1 of 3): As nitrogen is in fifth group in periodic table therefore it will have five electrons in the valance shell in which three electrons are unpaired because it needs three electrons to complete its outermost shell. Therefore in case of N2, each nitrogen atom will share three electron...
In order to come up with this answer, you first need to know the number of valence electrons for Nitrogen. Since N is a member of the Group 5A (based on the periodic table), the number of electrons in its outermost shell must be 5. Here is the electron dot structure for a single N atom: The total number of valence electrons between the two N atoms is 10 e^-.
N2 And Co2 Lewis Structure, Multiple Bonds in Covalent Bonding dummies, CH2N2 Lewis Structure: How to Draw the Lewis Structure for, What is the Lewis dot diagram for carbon dioxide? Quora, Bond Length and Bond Energy
How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
For diatomic nitrogen, the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms: This triple bond is very strong. The strength of the triple bond makes the N 2 molecule very stable against chemical change, and, in fact, N 2 is considered to be a chemically inert gas .There is a relationship between the ...
Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i...
Lewis Dot Structure for Compounds. Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows ...
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell.As per the molecule N2, it has two atoms of Nitrogen.
The dot structure of Na+1 is Na+1 . The dot structure of O-2 is O-2. Note that Na is in group 1 and should lose 1 electron while O is in group 6 and should gain 2 electrons. ionic compounds Make certain it's ionic: one atom must be from groups 1-3, the other from groups 4-7 (including H).
















0 Response to "35 lewis dot diagram n2"
Post a Comment