38 adiabatic process pv diagram
The combustion process inside a car engine is essentially adiabatic for this reason. An isobaric process is a process that occurs at constant pressure. We then have W = P(V 2 - V 1). If the pressure of an ideal gas is kept constant, then the temperature must increase as the gas expands. (PV/T = constant.) PV g = constant where g = CP/CV Because PV/T is constant (ideal gas): V g-1 T = constant (for adiabatic) P V Adiabat Isotherms. Proof of PV g =constant (for adiabatic process) 1)Adiabatic: dQ = 0 = dU + dW = dU + PdV 2) U only depends on T: dU = n CV dT (derived for constant volume, but true in general)
pV γ pVγ 1 constant 2 2 1 1 1 = = T Vγ− T V γ− pV =nRT During an adiabatic expansion process, the reduction of the internal energy is used by the system to do work on the environment. During an adiabatic compression process, the environment does work on the system and increases the internal energy. Ideal gas: adiabatic process (contd)

Adiabatic process pv diagram
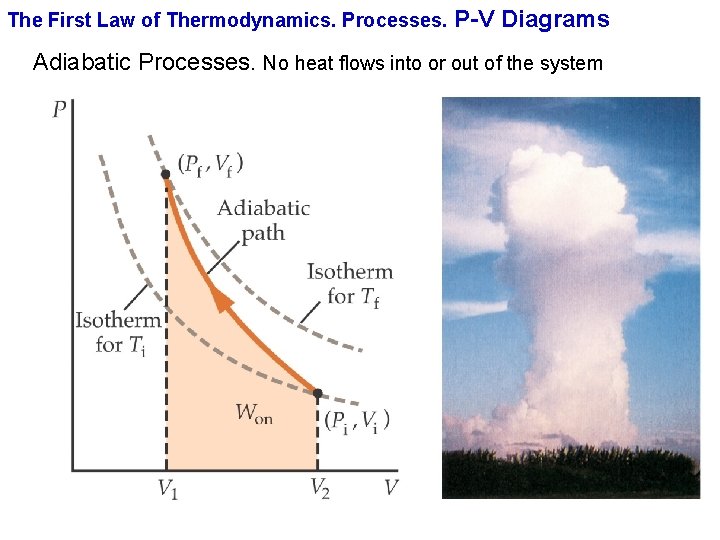
Processes, Adiabatic Process, PVT Relationship, PV diagram, TS diagram, Change in Internal Energy, Change in Entropy, Work done, Heat Transferred, Constant Temperature Process, PVT Relationship, PV diagram, TS diagram, Change in Internal Energy, Change in Entropy, Work The compression and expansion events are modeled thermodynamically as adiabatic processes, meaning it is assumed that no heat is transferred to or from the gas during these events. An adiabatic thermodynamic process is an isentropic (constant entropy) process. The area of the P-V diagram in Figure 1 bounded by 1-2-3-4-1 is the adiabatic power. shown in the pV-diagram of Fig. P20.34. Process 1!2 is at constant volume, process 2!3 is adiabatic, and process 3!1 is at a constant pressure of 1.00 atm. The value of for this gas is 1.40. a) Find the pressure and volume at points 1, 2, and 3. b) Calculate Q, W, and U for each of the three processes. c) Find the net work done by the gas in ...
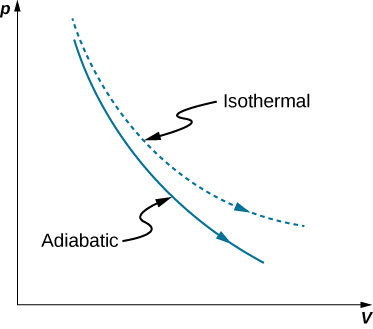
Adiabatic process pv diagram. here , we can conclude that the slope of PV diagram of Polytropic process is negative and greater than isothermal process but less than Adiabatic process. Because , 1 < N < ¥ Therefore the P-V diagram of Polytropic process will be like : The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For an ideal gas, an adiabatic process is a reversible process with constant entropy. The mathematical representation of the adiabatic process is ΔQ=0 Answer (1 of 2): On a PV diagram, only adiabatic and isotherm processes have asymptotic shapes. An example case for the curves for the two processes is shown here: It is not possible to just look at the shape of one curve and predict if it adiabatic or isotherm, because they look very similar. ... Difference between reversible and irreversible adiabatic process in PV diagram. Ask Question Asked 4 years, 11 months ago. Active 4 years, 9 months ago. Viewed 4k times 0 $\begingroup$ let's say we've got a carnot-cycle in a pressure volume diagram with the following processes: 1 -> 2: reversible isothermal ...
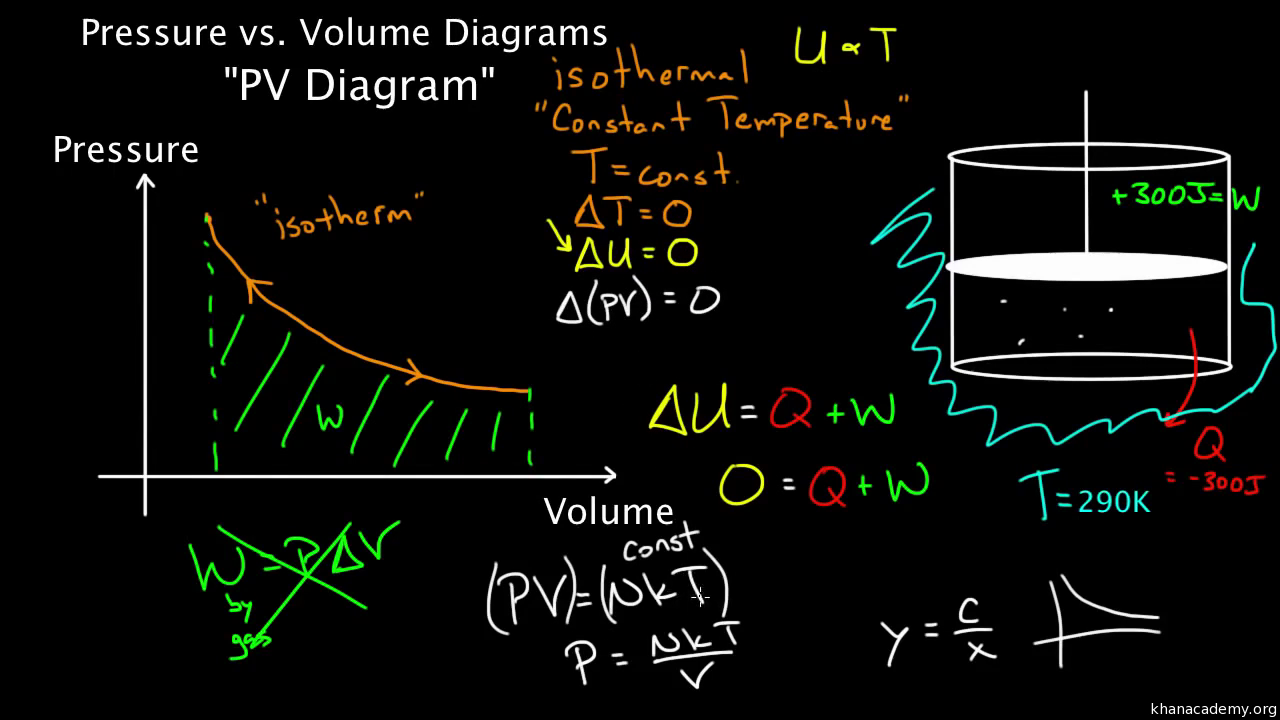
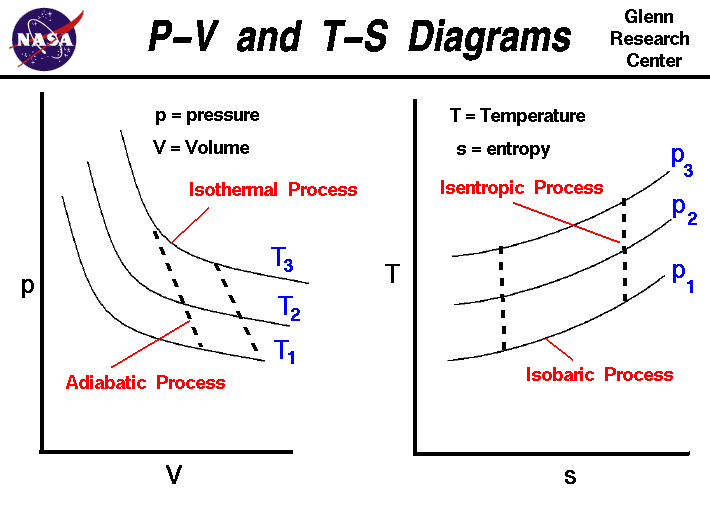
So on a PV diagram, an isothermal process is gonna look something like this, it's gonna curve like 1/x and it can be an isothermal expansion if volume increases or an isothermal compression if volume decreases. So the actual shape of the line drawn on a PV diagram for an isothermal process is sometimes called an isotherm and they look like that. Otto Cycle is a constant volume cycle on which petrol and gas engines work. The Otto cycle consists of 4 processes and are as follows. Process 1-2: Reversible Adiabatic Compression or Isentropic Compression. Process 2-3: Constant Volume heat supply. Process 3-4: Reversible Adiabatic Expansion or Isentropic Expansion. During an adiabatic process no heat is transferred to the gas, but the temperature, pressure, and volume of the gas change as shown by the dashed line. As described on the work slide, the area under a process curve on a p-V diagram is equal to the work performed by a gas during the process. On the right of the figure we have plotted the ... (a) Isothermal process: (b) Adiabatic process : PV diagram for adiabatic expansion and adiabatic compressior (c) isobaric process : PV diagram for an isobaric process (d) Isochoric process : (a) increased pressure and (b) decreased pressure
While a truly adiabatic process is not possible, near adiabatic conditions can be achieved by reducing the volume rapidly or using a very well insulated container. When an ideal gas undergoes an adiabatic expansion or compression, the adiabatic equation can be used: $$ pV^{\gamma} = \text{constant} $$ The PV diagram for an adiabatic process show a special result. • An adiabatic process looks very much like an isothermal process, but it drops off to a lower point. This means it is on a different isotherm. Remember, isotherms are just lines that show where the temperature stays constant. gas expands during an adiabatic process, because U is decreasing. Adiabatic and isothermal (quasi-static) processes are reversible , because there is no heat flow from hot to cold. This is always true, not just for ideal gases. “Quasi-static”means slow enough that the system is always near thermal equilibrium. We’ll discuss this more ... Adiabatic is a Greek word in which ‘a’ means ‘not’, ‘dia’ means ‘through’ and ‘bait’ means ‘hot’.So in short adiabatic is a system that does not allow heat to pass through it. Definition: It is the thermodynamic process in which there is a change in pressure, volume, and temperature of the system, but there is no exchange of heat between the system and surroundings.
Isothermal process on p-V, T-V, and p-T diagrams ... ideal gas law: pV = nRT Consider the p-V diagram below in which the system evolves from a ... Adiabatic processes reversible a = (p 1, V 1, T 1) b = (p 2, V 2, T 2) a p p 1 p 2 V 1 V 2 b V T 1 T 2 isotherms adiabat pV ...
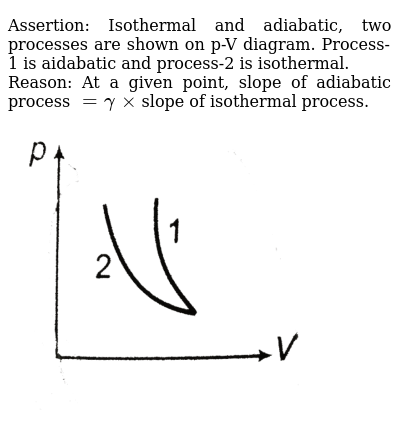
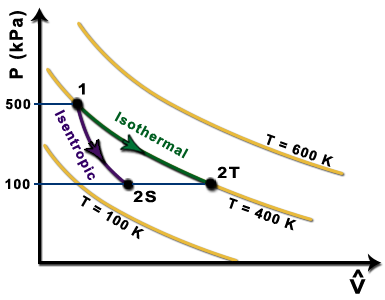
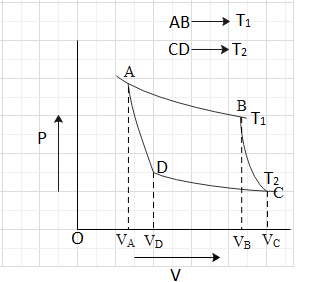
Assertion Isothermal And Adiabatic Two Processes Are Shown On P V Diagram Process 1 Is Aidabatic And Process 2 Is Isothermal Reason At A Given Point Slope Of Adiabatic Process Gammaxxslope Of Isothermal Process Img
Adiabatic. Comparing isothermal and adiabatic processes. In this simulation, you can look at the difference between a constant temperature (isothermal) process and an adiabatic process. The paths look somewhat similar on the P-V diagram, but you should notice clear differences. Note that an isothermal process has no change in temperature, so the change in internal energy is zero, but in an adiabatic process the heat transferred is zero.
Breaking the Question Down Into Parts to Understand It Better. To make it easier to arrive at an answer, it is often helpful to take a multi-part question and break it down into simpler parts.
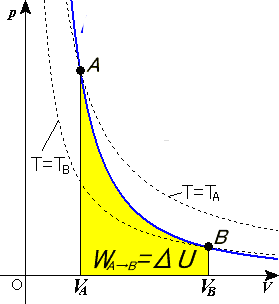
A process has to be sudden and quick to be adiabatic. Here the graph above shows the P-V diagram for a adiabatic process.During an adiabatic process from volume V 1 to V 2,the work done for an increase in volume is dV. dV=PdV ——————- (1) Work done when the gas expands from volume V 1 to V 2 is given by
Suppose in an adiabatic process pressure and volume of a sample of gas changes from (P 1, V 1) to (P 2, V 2) then we have. Thus, P = K V γ P = K V γ. Work done by gas in this process is. W = ∫ V 2 V 1 P dV W = ∫ V 1 V 2 P d V. where limits of integration goes from V 1 to V 2. Putting for P = K V γ P = K V γ, and integrating we get,
The equation for an adiabatic process expressed as a function of pressure and volume is:. Where γ is the heat capacity ratio (or adiabatic index), that in the case of a monatomic ideal gas is given by:. Pressure and volume in state 1 are knowns; on the other hand, the volumes in states 3 and 2 are the same (see the thermodynamic cycle in the PV diagram), so we can use the adiabatic process ...

Jee Main 2021 Online 18th March Morning Shift Jee Main Year Wise Previous Years Questions Examside Com
Visit us (http://www.khanacademy.org/science/healthcare-and-medicine) for health and medicine content or (http://www.khanacademy.org/test-prep/mcat) for MCAT...
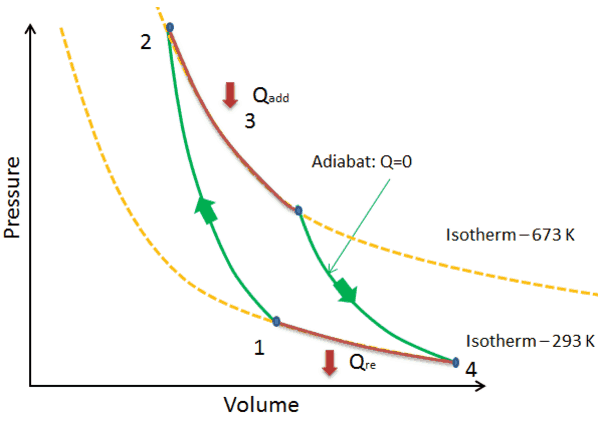
Process 4-5 :-Reversible expansion for reversible adiabatic process. `Q_{4-5}=0` By expansion of air from 4 to 5, temperature of air decreases from `T_{4}` to `T_{5}`. Hence change in internal energy is given by, ... Process, Formula, Pv & Ts Diagram, Application [with Pdf]” ...
The PV diagram for an adiabatic process is also called adiabat Note that the PV diagram for isothermal (Figure 8.25) and adiabatic (Figure 8.30) processes look similar. But actually the adiabatic curve is steeper than isothermal curve. We can also rewrite the equation (8.35) in terms of T and V.
When we subject the gas to these thermodynamics processes, the pressure and volume of the gas can change. A convenient way to visualize these changes in the pressure and volume is by using a Pressure Volume diagram or PV diagram for short. Each point on a PV diagram corresponds to a different state of the gas.
adiabatic no heat exchange with the environment; adiabatic has a complex greek origin that means "not+through+go": α + ∆ια + βατός [a + dia + vatos] examples: "fast" processes, forcing air out through pursed lips, bicycle tire pump; PV diagram is a "steep hyperbola"
Equilibrium Processes In Gases Slow Almost Unchanging With Respect To The Typical Relaxation Times Of A System Equilibrium Processes Are Represented Ppt Download
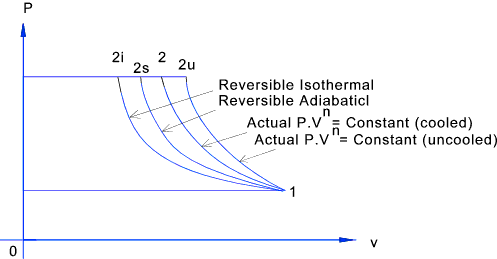
However, the former exist only as theoretical tools to study the latter. Thus, reversible adiabatic processes involve ideal gases, and lack friction and any other eventuality that causes heat transfer between the system and its surroundings. Consider for example the PV diagram for the reversible adiabatic process above.
In an isothermal process the initial room temperature is constant. W = 1 × 8.3 × 300 × ln(2) = 1.7kJ (b) Comparing all three processes, we see that the work done in the isobaric process is the greatest, and work done in the adiabatic process is the least. (c) The PV diagram is shown in the Figure.
This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process. For example, if an ideal gas makes a quasi-static adiabatic transition from a state with pressure and volume and to a state with and then it must be true that . The adiabatic condition of can be written in terms of other pairs of thermodynamic variables by combining it with the ideal gas law.
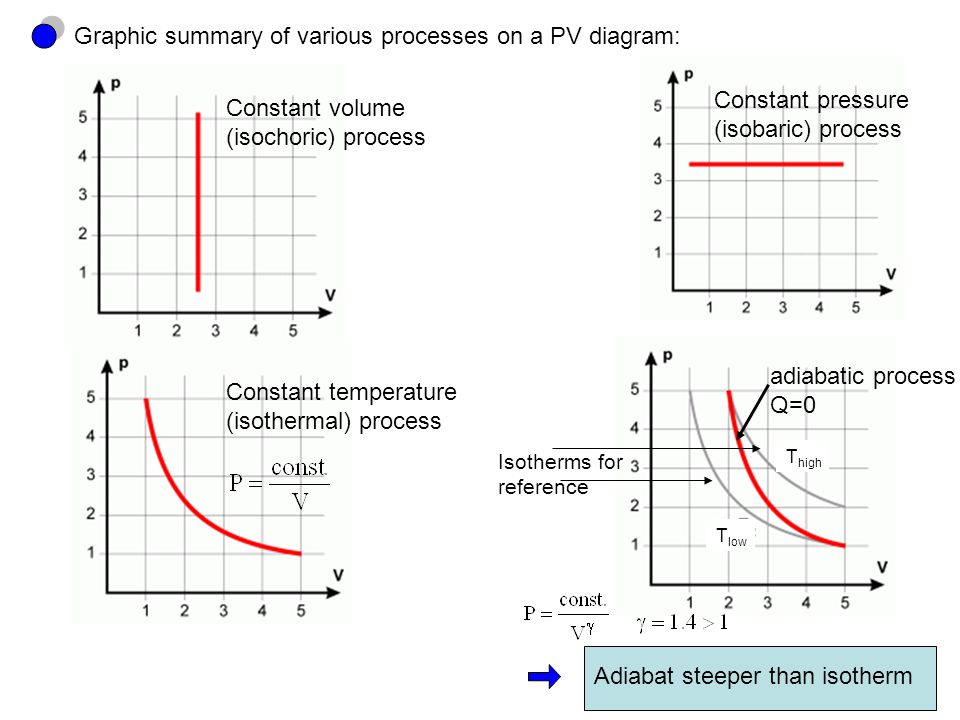
The fundamental thermodynamic processes modelled on PV diagrams (isochoric, isobaric, and isothermal processes) all follow the ideal gas law except for adiabatic processes—which will be discussed in detail on its main page. The following are the examples of each process modelled on the PV diagram.

Jee Ma The Pt Diagram For An Ideal Gas Is Shown In The Figure Where Ac Is An Adiabatic Process Find The Corresponding Pv Diagram
shown in the pV-diagram of Fig. P20.34. Process 1!2 is at constant volume, process 2!3 is adiabatic, and process 3!1 is at a constant pressure of 1.00 atm. The value of for this gas is 1.40. a) Find the pressure and volume at points 1, 2, and 3. b) Calculate Q, W, and U for each of the three processes. c) Find the net work done by the gas in ...
The compression and expansion events are modeled thermodynamically as adiabatic processes, meaning it is assumed that no heat is transferred to or from the gas during these events. An adiabatic thermodynamic process is an isentropic (constant entropy) process. The area of the P-V diagram in Figure 1 bounded by 1-2-3-4-1 is the adiabatic power.
Processes, Adiabatic Process, PVT Relationship, PV diagram, TS diagram, Change in Internal Energy, Change in Entropy, Work done, Heat Transferred, Constant Temperature Process, PVT Relationship, PV diagram, TS diagram, Change in Internal Energy, Change in Entropy, Work
Neet Nta National Eligibility Cum Entrance Test Medical Physics Work In Thermodynamic Process And Pv Diagrams Thermodynamics Study Material Page 1 Of 2 Doorsteptutor

The First Law Of Thermodynamics And Some Simple Processes Une Physics For The Health Professions Openstax Cnx
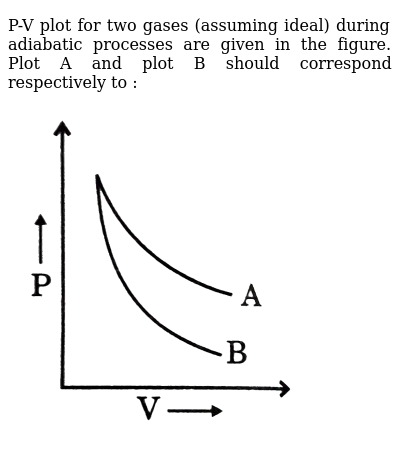
P V Plot For Two Gases Assuming Ideal During Adiabatic Processes Are Given In The Figure Plot A And Plot B Should Correspond Respectively To Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images Grb Phy Chm P2 V03 Qb C13 E01 040 Q01 Png













0 Response to "38 adiabatic process pv diagram"
Post a Comment