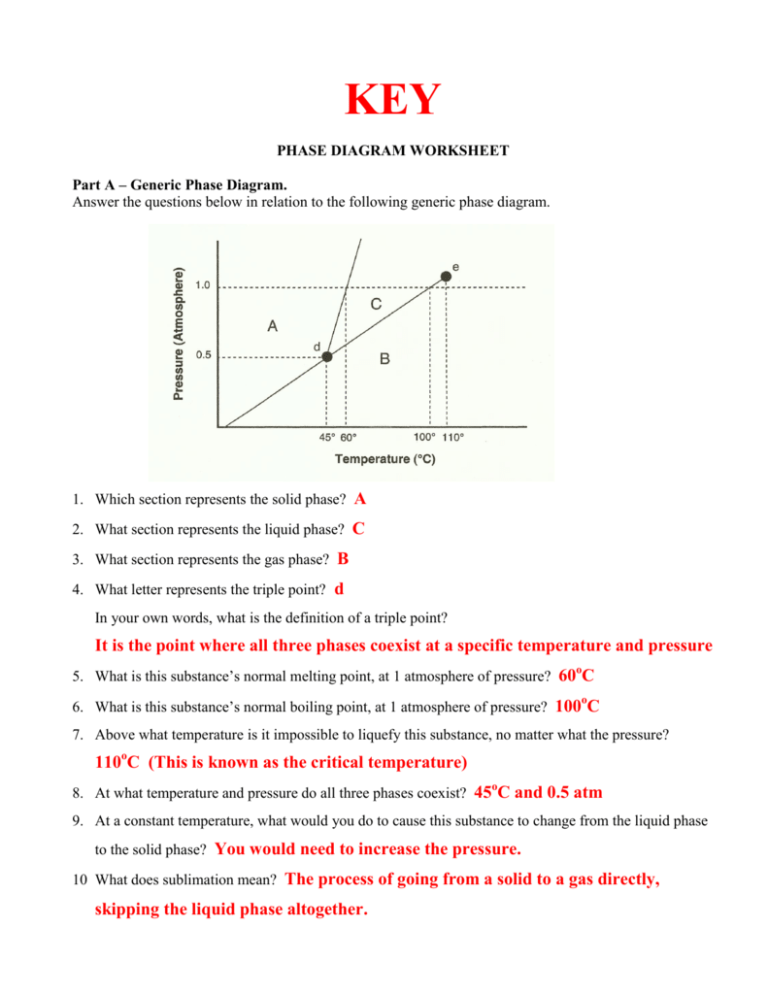
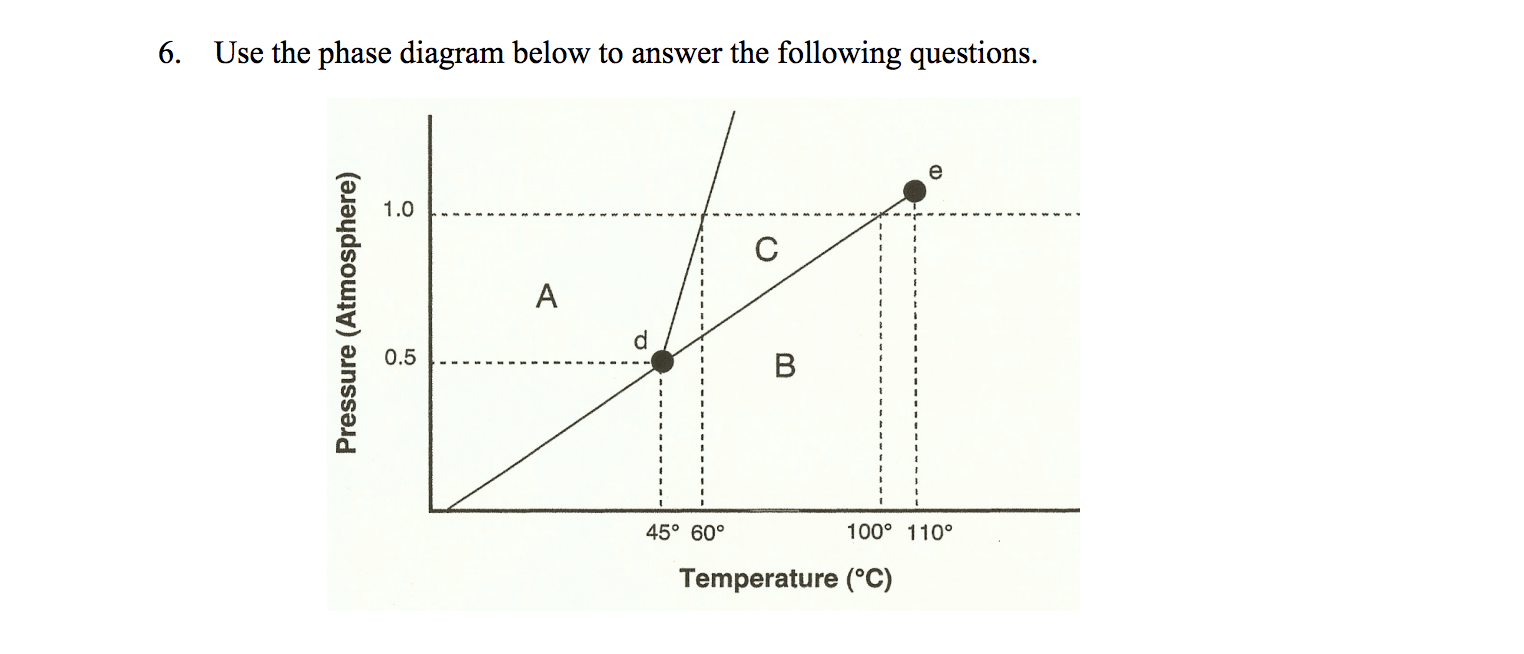
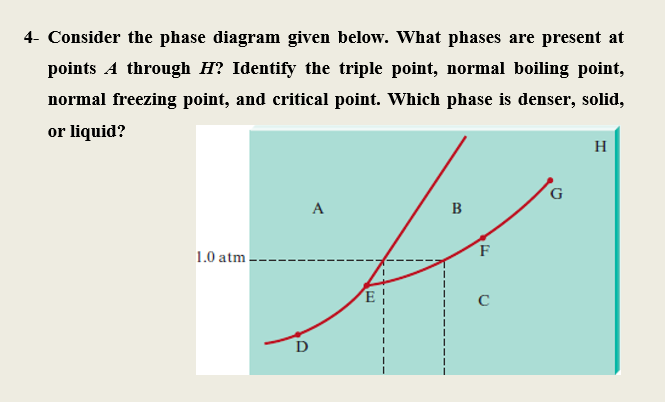
36 examine the following phase diagram and determine what phase(s) exists at point a.
Examine the following phase diagram and determine what phase exists at point F. Examine the following phase diagram and determine what phase exists at point F. A)Vapor + Liquid. B)Vapor. C)Liquid. D)Solid. E)Supercritical fluid. Categories. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. A) Bo(s) has a lower density than Bo(l) B) the triple point for Bo is at a higher temperature than the melting point for Bo C) Bo changes from a solid to a liquid as one follows the line from C to D
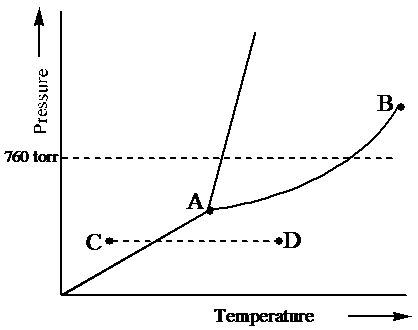
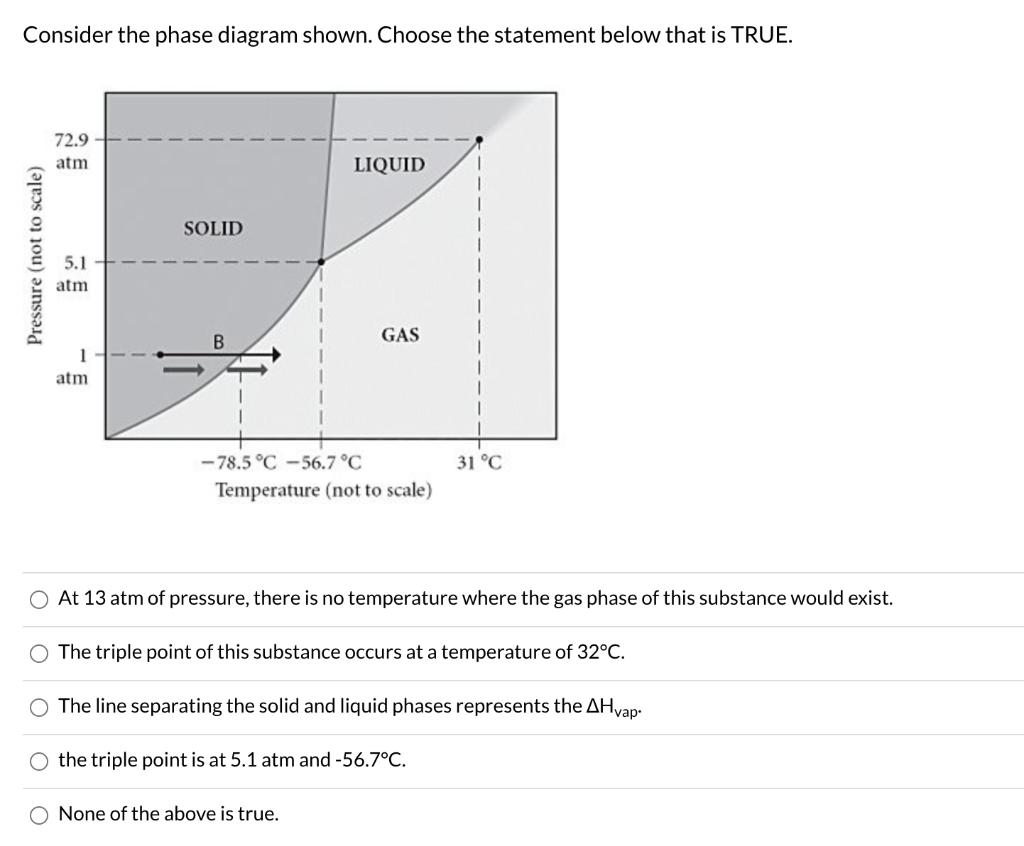
7) What is the phase (s, l, g) of a substance at 0.5 atm and 100 0 C? 8) What is the phase (s, l, g) of a substance at 1.5 atm and 50 oc? 9) What is the phase (s, l, g) of a substance at 1.5 atm and 200 oc? 10) What is the phase (s, l, g) of a substance at 1.5 atm and 800 oc? 11) What is the condition of the triple point of this substance? T=

Examine the following phase diagram and determine what phase(s) exists at point a.
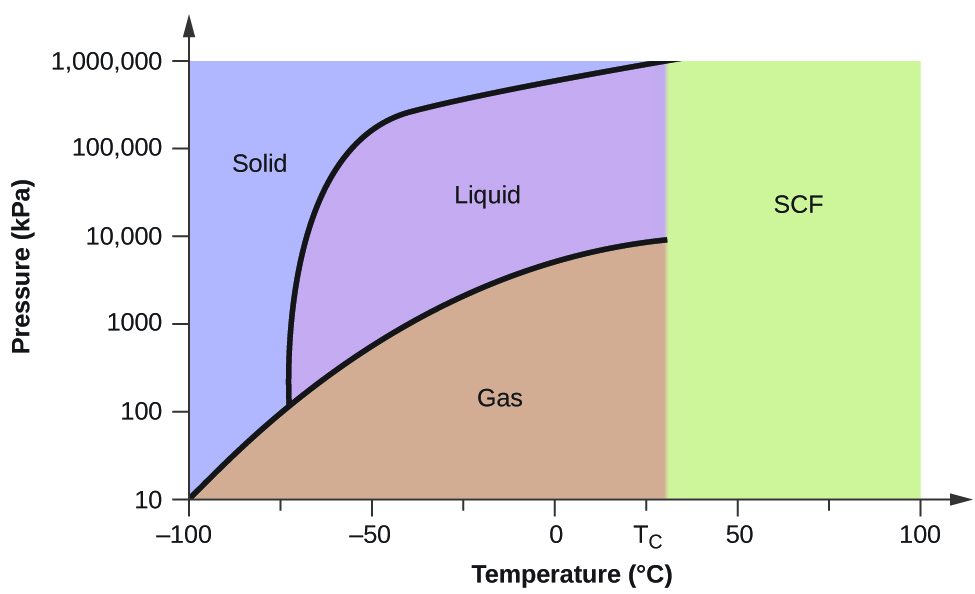
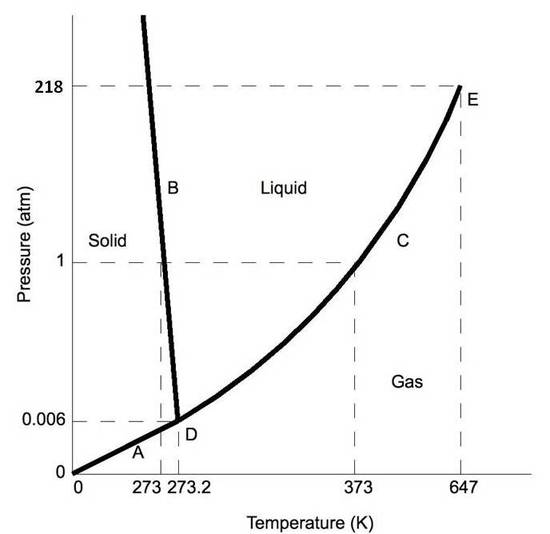
A typical phase diagram for a pure substance is shown in Figure 1. ... At pressures lower than the triple point, water cannot exist as a liquid, ... ... the next question prevents changes to this answer, Question 2 Examine the following phase diagram and determine what phase exists at point F. Pressure . We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled “ice.”. Under these conditions, water exists only as a solid (ice).
Examine the following phase diagram and determine what phase(s) exists at point a.. Transcribed image text: 1 pe QUESTION 19 Examine the following phase diagram and determine what phase exists at point F. Teeper INote the phase diagram does ... Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid. 2. gas. 3. liquid. 4. solid. 5. supercritical fluid. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. SEE QUESTION 10 Image A) Bo(s) has a lower density than Bo(l). B) The triple point for Bo is at a higher temperature than the melting point for Bo. C) Bo changes from a solid to a liquid as one follows the line from C to D. Problem: Examine the following phase diagram and identify the feature represented ... Critical Point: the point at which the liquid and gas phases coexist.1 answer · Top answer: Critical point
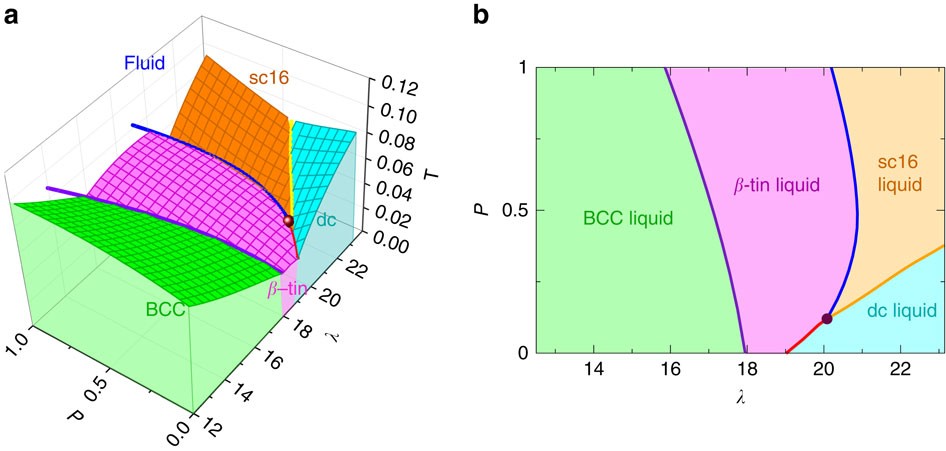
(Fig. 9.3(b) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash (Ed.), ASM International, Materials Park, OH, 1991.) Phase Diagrams: weight fractions of phases wt% Ni 20 1200 1300 T(°C) L (liquid) α L + α (solid) l i q u i d u s s o l i d u s 30 40 50 L + α Cu-Ni system TA A 35 Co 32 CL B TB D TD tie line 4 Cα 3 R S At TB ... Examine the following phase diagram and determine what phase(s) exists at point a.. Examine the phase diagram for the substance bogusium bo and select the correct statement. Examine the following phase diagram and identify the feature represented by point a and point b. Temperature vapor liquid vapor liquid solid supercritical fluid e. Chemistry questions and answers. 12) Examine the following phase diagram and identify the feature represented by point A. 760 torr Temperature A) melting point B) critical point C) triple point D) sublimation point E) boiling point 13) In hydrogen iodide are the most important intermolecular forces A) B) C) dipole-dipole forces London ... Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
Question: examine yhe following phase diagram and determine what phase exists at point F. This problem has been solved! See the answer ...Missing: s) | Must include: s) Chemistry questions and answers. Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below. This substance is a at 25°C and 1.0 atm. 1.5 P (atm) 1.0 0.5T -10 0 10 20 30 40 50 60 70 T ('C) A ... We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled “ice.”. Under these conditions, water exists only as a solid (ice). ... the next question prevents changes to this answer, Question 2 Examine the following phase diagram and determine what phase exists at point F. Pressure .

On A Phase Diagram There Is A Triple Point Where Solids Liquids And Gasses All Exist At The Same Temperature And Pressure If The Temperature Is Decreased But The Pressure Is Increased
A typical phase diagram for a pure substance is shown in Figure 1. ... At pressures lower than the triple point, water cannot exist as a liquid, ...
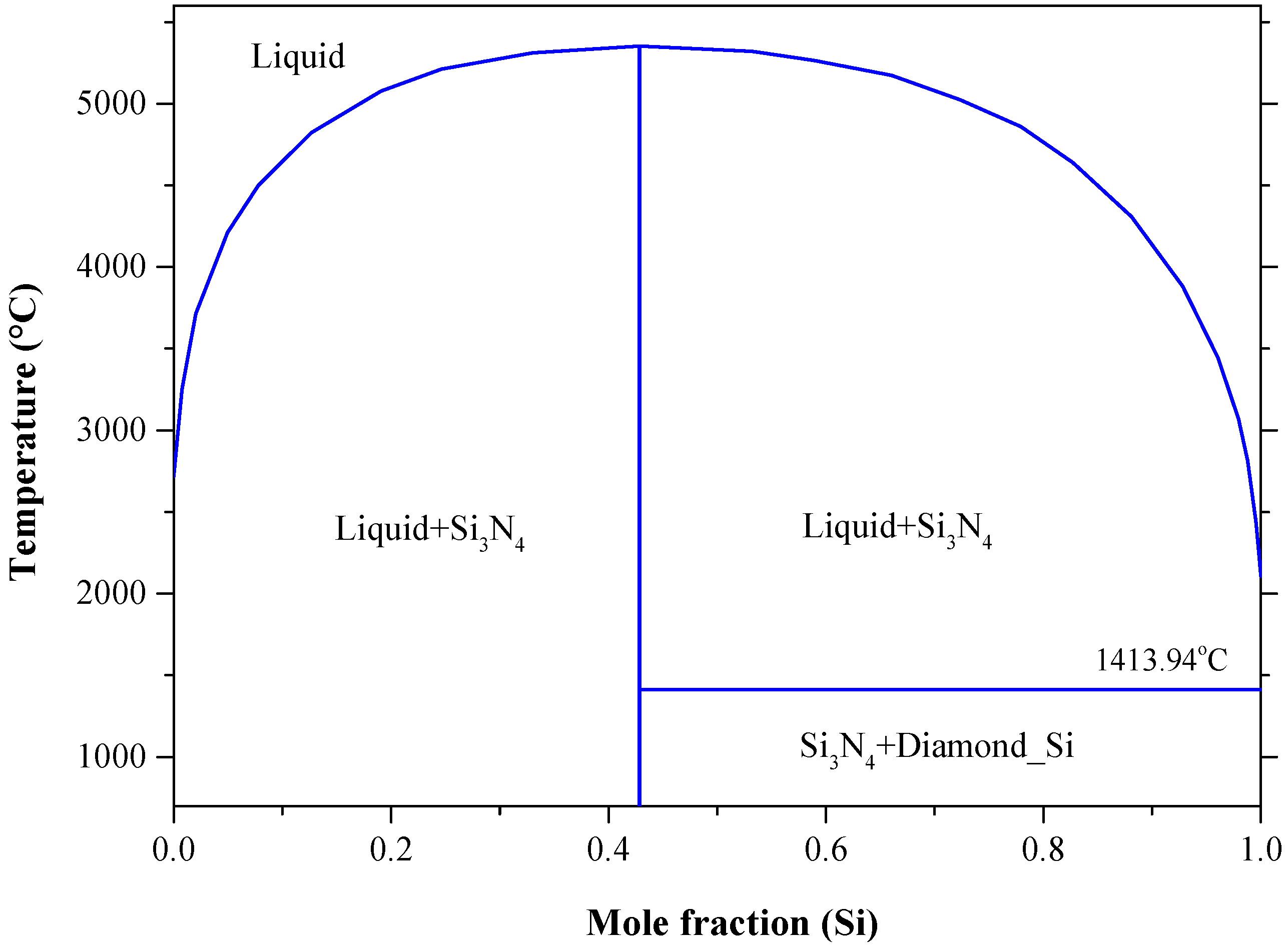
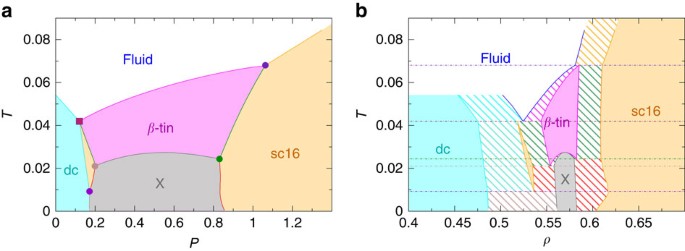
Materials Free Full Text Binary Phase Diagrams And Thermodynamic Properties Of Silicon And Essential Doping Elements Al As B Bi Ga In N P Sb And Tl Html

















0 Response to "36 examine the following phase diagram and determine what phase(s) exists at point a."
Post a Comment