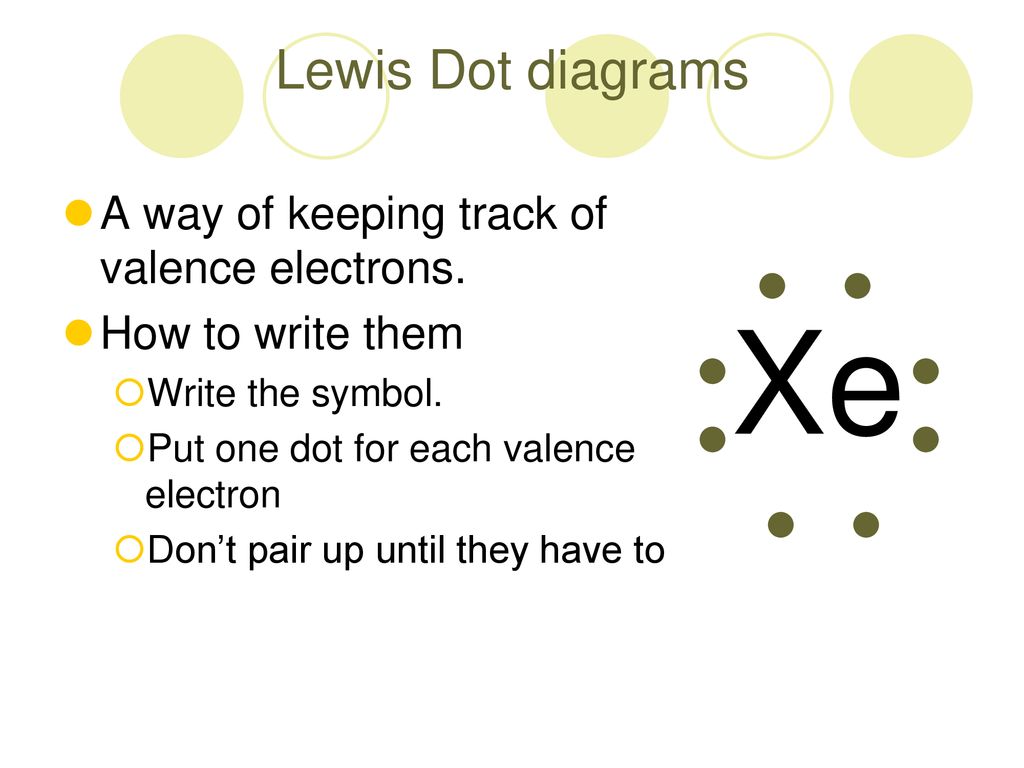
36 electron dot diagram for xenon
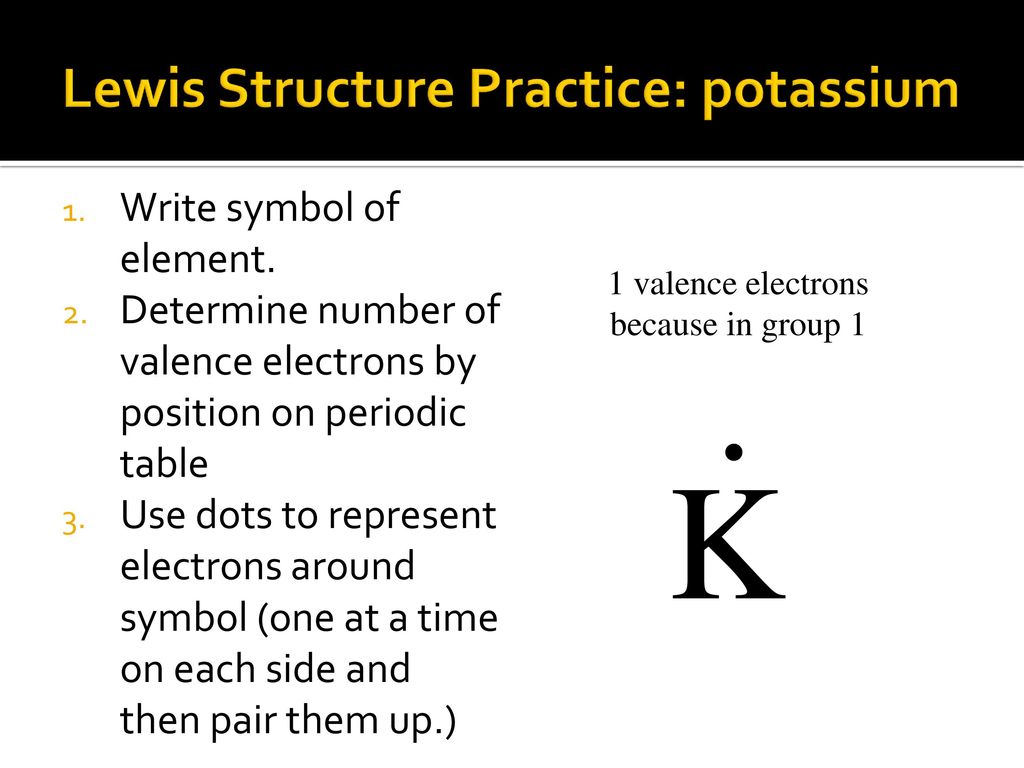
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... Xef2 Lewis Structure, Polarity, Hybridization and shape. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). Out of these compounds, XeF2 is the most ...
Comprehensive information for the element Xenon - Xe is provided by this page including scores of Atomic Structure of Xenon Electron Dot Model. for XeF2. Step-by-step tutorial for drawing the Lewis Structure for XeF2. for the molecule. Remember that Xenon can have more than 8 valence electrons.A covalent bond, also called a molecular bond, is a ...

Electron dot diagram for xenon
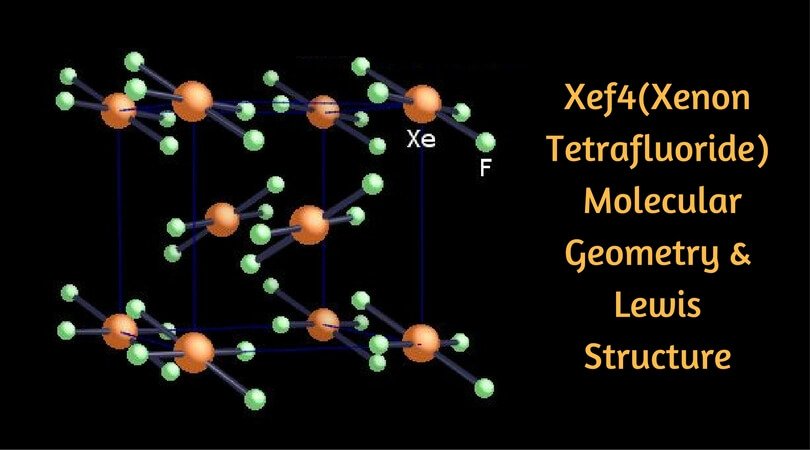
How to Draw a Lewis Structure for XeO3 xenon trioxide?Lewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Sub... In the XeF4 Lewis structure diagram, the xenon atom can be the center atom of the molecule. As a result, central xenon in the XeF4 Lewis structure, with all four fluorine arranged in the square planar geometry. Add valence electrons around the fluorine atom, as given in the figure. Step-3: Lewis dot Structure for XeF4 generated from step-1 and ... Lewis Dot of Xenon Oxytetrafluoride. XeOF 4. Back: 70 More Lewis Dot Structures. Xe does not follow the octet rule. It actually bonds. It will hold more than 8 electrons. Xenon having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons.
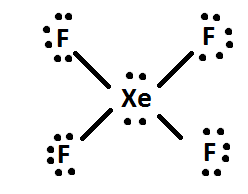
Electron dot diagram for xenon. The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence electrons. Refer to the related link for an illu. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can . A step-by-step explanation of how to draw the XeF2 Lewis Dot Structure (Xenon difluroide).For the XeF2 structure use the periodic table to find the total num... Xenon having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons. XeF 2 is dsp 3 hybridized and contains 3 lone pair and 2 bonding pairs of valence electrons around the Xenon. The VSEPR predicts the linear shape. XeF2 Lewis Structure. Lewis Structure, also known as electron dot structure, is an essential model of chemical bonding where we use the valence electron concept to schematically sketch a two-dimensional figure of a given molecule. We use dots to represent outer shell electrons and lines to represent the bond type. Xenon is an inert gas element.
In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure. The Lewis structure for XeF 4 requires you to place more than 8 valence electrons on Xe. Xenon (Xe) can have more than 8 valence electrons in your Lewis structure. Hydrogen (H) only needs two valence electrons to have a full outer shell. XeO4 Lewis Structure, Geometry, Hybridization, and Polarity. XeO4 or Xenon Tetraoxide is a chemical compound made up of Xenon and Oxygen. It is prepared by treatment of barium perxenate with anhydrous sulphuric acid. It has a molar mass of 195.29 g/mol. It is exceptional for being a stable compound of a noble gas comprising of Xenon in its ... The total valence electron is available for drawing the XeO4 Lewis structure is 32. The steric number of Xenon central atom in the XeO4 molecule is 4, thus, it forms Sp 3 hybridization. The net dipole moment of XeO4 is zero, hence, it is a nonpolar molecule. The molecular geometry of XeO4 is tetrahedral because the central atom Xenon is ... Lewis Structure for CO 3 2-| Carbonate ion. Lewis structure of carbonate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of CO 3 2-.After finishing the lewis structure of CO 3 2-, there should be a -2 charge and it should be stabile structure.
A step-by-step explanation of how to draw the Xe Lewis Dot Structure.For the Xe structure use the periodic table to find the total number of valence electron... For the Lewis Structure first, we need to know the total number of the valence electron in the Xenon tetrafluoride molecule for that it needs to add the valence electron of Xenon and Fluoride atoms. From the periodic table, the xenon has 8 electrons in its valence shell and the fluoride atom has 7 electrons in its valence shell. XeF 4 (Xenon tetrafluoride) Lewis Structure. In XeF 4 (Xenon tetrafluoride) lewis structure, there are four sigma bonds and two lone pairs around xenon atom. Each fluorine atom has three lone pairs. In this tutorial, we will learn how to draw lewis structure of XeF 4 step by step.. Lewis structure of XeF 4. Xenon atom is the center atom and each fluorine atom has made a single bond with xenon ... Lewis Structure. To begin with the Lewis structure of the compound xenon tetrafluoride, it is quite essential to know the meaning of the same. It is a simplified symbolic representation of the electrons which lie in the valence shell of a molecule.
A step-by-step explanation of how to draw the XeBr4 Lewis Dot Structure.For the XeBr4 structure use the periodic table to find the total number of valence el...
Chemistry questions and answers. (a) (2 points) In the space provided below, draw a Lewis structure for the molecule xenon difluoride, XeF2. If there are any atoms with a nonzero formal charge, be sure to write the formal charge next to the symbol. (b) (2 points) C The electronegativity of Xe is 2.6. The electronegativity of F is 4.0.
Answer the following question regarding Lewis Structure for Covalent Molecule: What is incorrect about the following Lewis structure for xenon tetrafluoride, XeFA? :F: a. Too many electrons b. Too few electrons C. Incomplete octet d. Not showing resonance structures
Aug 31, 2021 — There are a total of 22 valence electrons in the Lewis structure for XeF2. The Lewis structure for XeF2 is a bit tougher since you have to ...
(d) Xenon can react with oxygen and fluorine to form compounds such as XeO 3 and XeF 4. In the boxes provided, draw the complete Lewis electron-dot diagram for each of the molecules represented below. XeO 3 XeF 4 One point is earned for each correct Lewis electron-dot diagram. Omission of lone pairs of electrons on the O or F atoms
Comprehensive information for the element Xenon - Xe is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. ... Xenon. Atomic Structure of Xenon. ... Electron Dot Model. Chemical Properties of Xenon. Electrochemical Equivalent: Electron ...
Oct 11, 2015 — Due to the atomic radius, the attraction of the outer valence electrons and the nucleus is not as strong, so Xenon can form bonds with other ...
The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence electrons. Refer to the related link for an illustration.
XeF4 Lewis Structure. Now that we know the valence electrons of Xenon Tetrafluoride, it will be easier for you to draw its Lewis structure. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms.
Aug 22, 2021 — How are xenon electronic points constructed? ... | There are a total of 22 valence electrons in the Lewis structure of XeF2. The Lewis structure ...
let's do one more example of constructing a Lewis diagram that might be a little bit interesting so let's say we want to construct the Lewis structure or Lewis diagram for xenon difluoride so pause this video and have a go of that all right now let's work through this together so first step we just have to account for the valence electrons xenon right over here it is a noble gas it has eight ...
Drawing the Lewis Structure for XeF 6. Viewing Notes: In the XeF 6 Lewis structure Xe is the least electronegative and goes at the center of the structure.; The Lewis structure for XeF 6 requires you to place more than 8 valence electrons on Xe.; Xenon (Xe) can have more than 8 valence electrons in your Lewis structure.

For The Lewis Structure Xeo4 Why Do We Have To Remove An Oxygen And Put Three Double Bonds To The Xenon When Octet Rule Is Met Study Com
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Feb 1, 2021 — You can here understand the Xe valence electrons representation. The dot diagram represents the numbers of Xenon valence electrons.
Lewis Dot of Xenon Oxytetrafluoride. XeOF 4. Back: 70 More Lewis Dot Structures. Xe does not follow the octet rule. It actually bonds. It will hold more than 8 electrons. Xenon having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons.
In the XeF4 Lewis structure diagram, the xenon atom can be the center atom of the molecule. As a result, central xenon in the XeF4 Lewis structure, with all four fluorine arranged in the square planar geometry. Add valence electrons around the fluorine atom, as given in the figure. Step-3: Lewis dot Structure for XeF4 generated from step-1 and ...
How to Draw a Lewis Structure for XeO3 xenon trioxide?Lewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Sub...
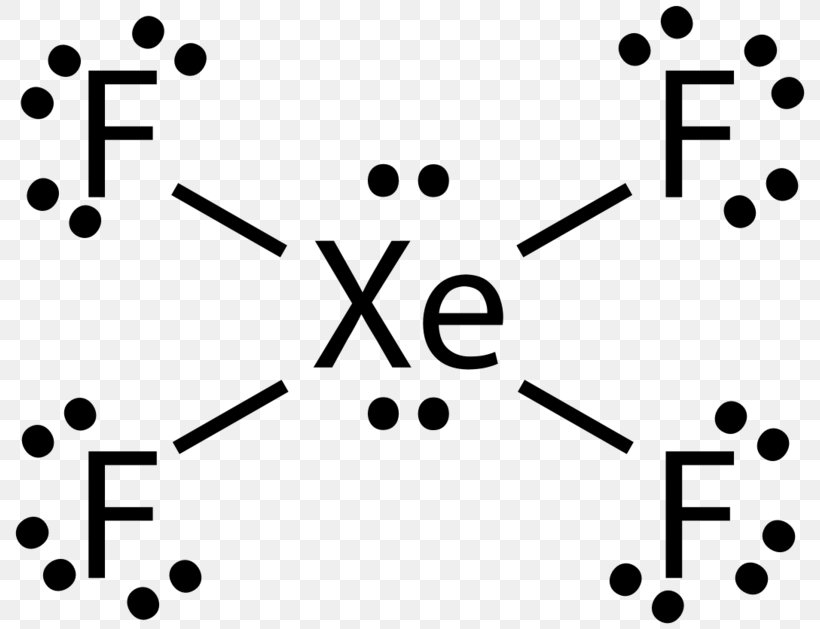
Lewis Structure Xenon Tetrafluoride Bromine Pentafluoride Sulfur Tetrafluoride Sulfur Hexafluoride Png 800x629px Watercolor Cartoon Flower Frame

















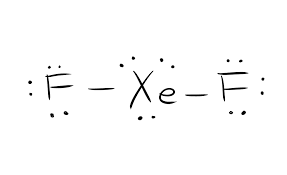
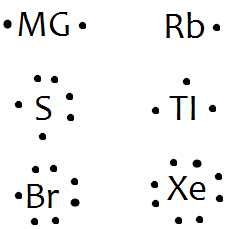

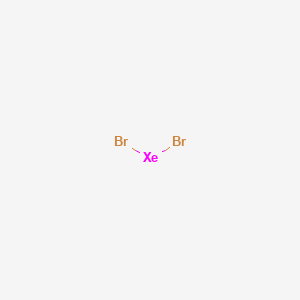
0 Response to "36 electron dot diagram for xenon"
Post a Comment