35 lewis dot diagram for pocl3
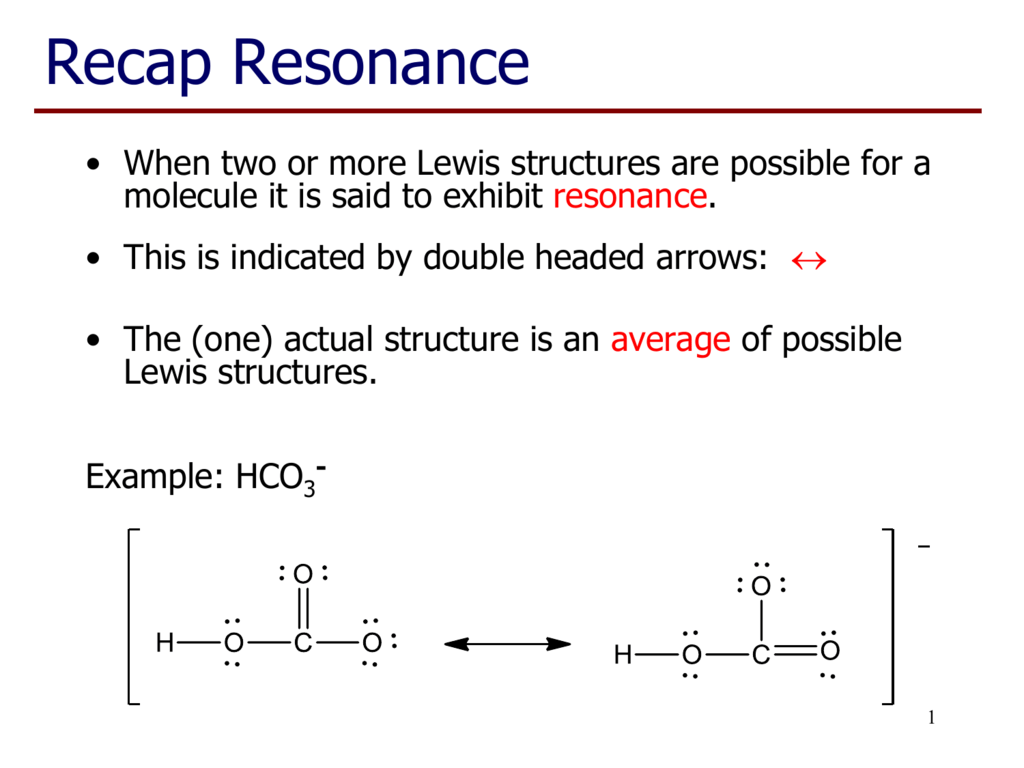
Lewis Structure of POCL3? - CHEMISTRY COMMUNITY Re: Lewis Structure of POCL3? You would have to leave the P-O bond as a double bond with two lone pair electrons because when you calculate the formal charge, it would be zero at every atom. If you left it as a single bond with three lone pairs, Oxygen would have a FC of -1 and P would have a formal charge of 1. How to draw Lewis dot structures | POCl3 Phosphorous ... how to draw Lewis dot structures| POCl3| lewis resonance structures| drawi for, the periodic of elements, pocl3 lewis structure, draw the lewis structure of pocl3, dot.cross structure of pocl3, Chemistry Net This chemistry blog is aimed mainly at senior high school students or first year university students. ...
POCl3 Lewis Structure - How to Draw the Lewis Structure ... A step-by-step explanation of how to draw the POCl3 Lewis Dot Structure (Phosphoryl chloride).For the POCl3 structure use the periodic table to find the tota...
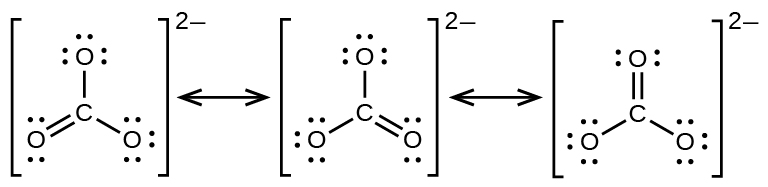
Lewis dot diagram for pocl3
POCl3 Lewis Structure, Molecular Geometry, Hybridization ... POCl3 Lewis Structure. The Lewis structure of any molecule helps understand the arrangement of atoms in the molecule, its bond formation, and the valence electrons participating in forming bonds. The valence electrons that take part in forming bonds are called bonding pairs of electrons, whereas those that do not form bonds are called lone ... How to draw PCl3 Lewis Structure? - Science Education and ... Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get ... PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.
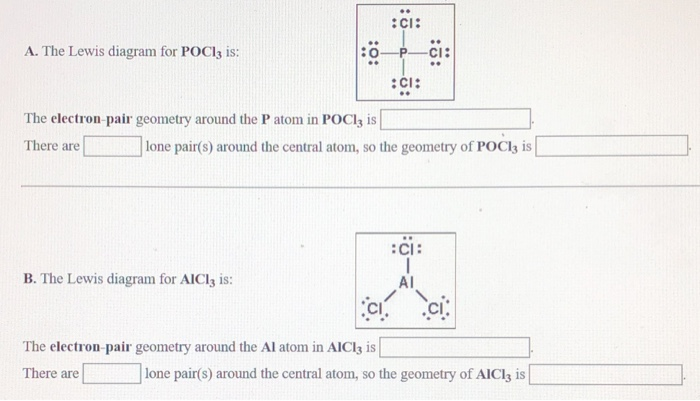
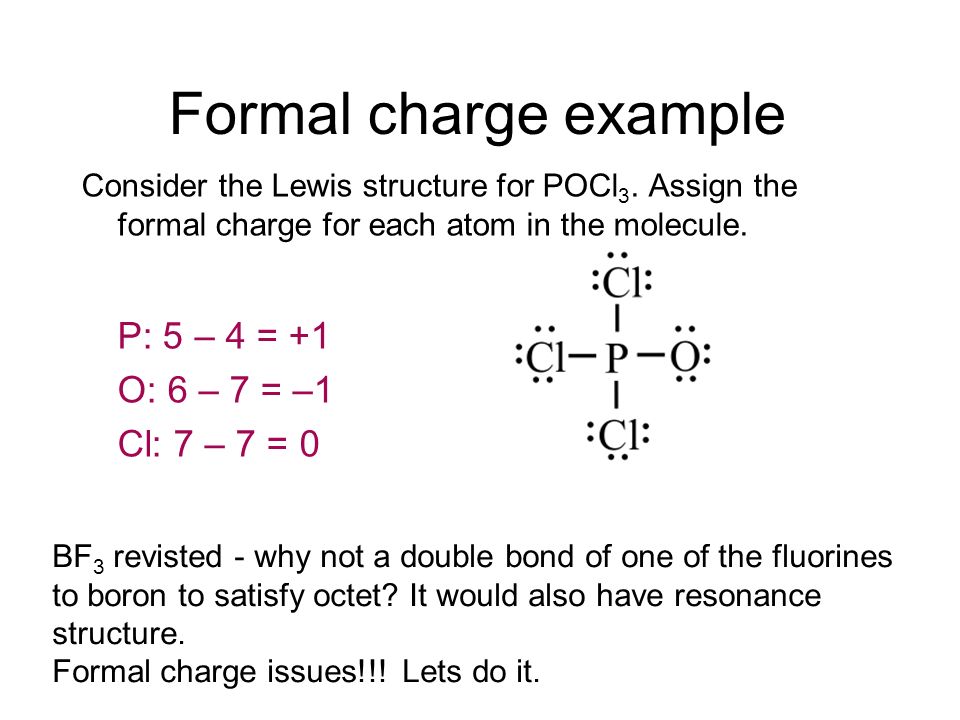
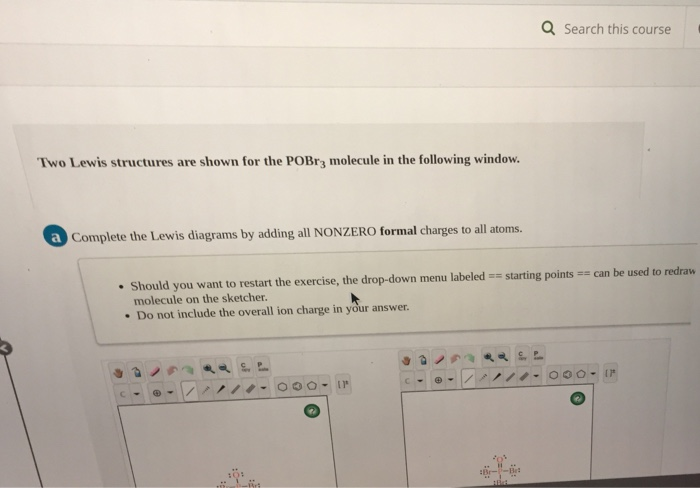
Lewis dot diagram for pocl3. Solved In the POCl3 molecule, the P atom is the central ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. tha …. View the full answer. Transcribed image text: In the POCl3 molecule, the P atom is the central atom. Draw a Lewis diagram for POCl3 in which all atoms have a formal charge of zero. PBr3 lewis dot structure, molecular geometry, polar or ... The molecular geometry or shape of PBr 3 is a Trigonal pyramid. The electron geometry for PBr 3 is Tetrahedral as its central atom has 4 regions of electron density. In the PBr 3 Lewis dot structure, a total of 10 lone pairs and 3 bond pairs are present. The hybridization of phosphorous in PBr 3 is sp 3. Phosphorus oxychloride | POCl3 - PubChem Phosphorus oxychloride | POCl3 or Cl3OP | CID 24813 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ... Solved :CI: A. The Lewis diagram for POCl3 is: :Ci: The ... The Lewis diagram for POCl3 is: :Ci: The electron-pair geometry around the P atom in POCl is There are lone pair (s) around the central atom, so the geometry of POCl3 is :CI: B. The Lewis diagram for AlCl, is: AI Cl Cl The electron-pair geometry around the Al atom in AICl3 is There are lone pair (s) around the central atom, so the geometry of ...
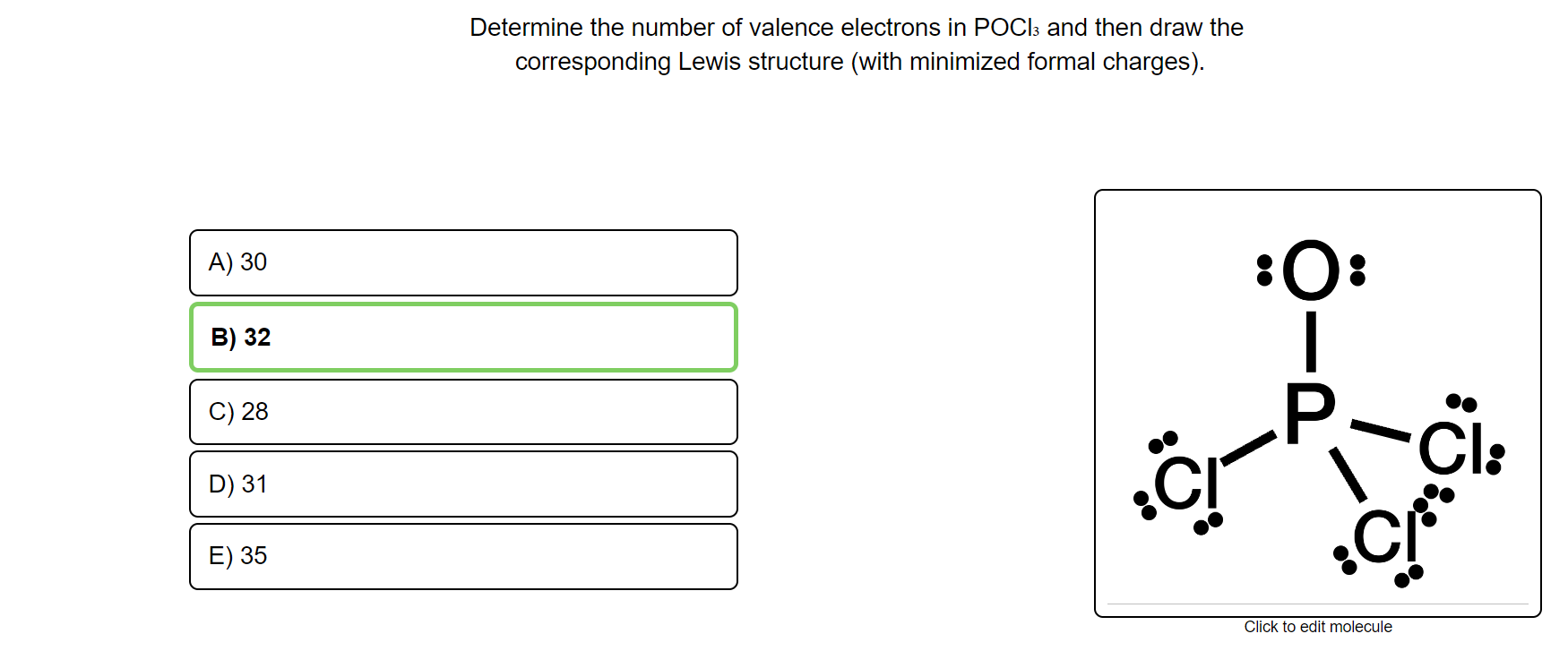
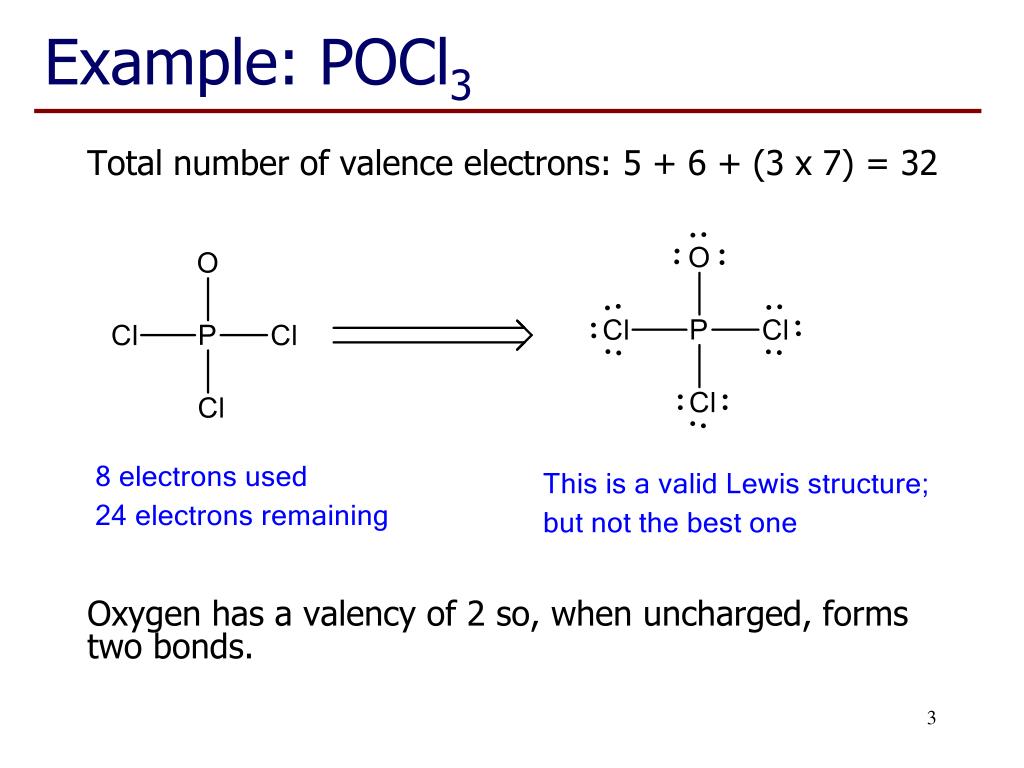
POCl3 Lewis Structure: How to Draw the Dot Structure for POCl3 In the POCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. In the Lewis structure for POCl 3 there are a total of 32 valence electrons. If you check the formal charges for POCl 3 you'll find that you need a double bond between the Phosphorous and Oxygen atom in order to have the formal charges equal zero. OneClass: Phosphoryl chloride, POCl3, has the skeleton ... Get the detailed answer: Phosphoryl chloride, POCl3, has the skeleton structure. Write (a) A Lewis structure for POCl3 following the octet rule. Calculate Lewis Dot Structure For Phosphorus Trichloride - drawing easy Drawing pcl3 lewis structure is very easy to by using the following method. Source: kovodym.blogspot.com. A o d o o o o which is the correct lewis dot structure for phosphorus trichloride (pci3) with the p as the central atom. Draw the lewis structure for phosphorus trichloride, nh3. Source: galvinconanstuart.blogspot.com B3.2 Extra VSEPR Practice.doc - B3.2: Extra VSEPR Practice ... View B3.2 Extra VSEPR Practice.doc from CHEM 20 at University of Calgary. B3.2: Extra VSEPR Practice Compound CH3SH PCl3 SiCl4 SBr2 H2S CH3CH3 CH2O Lewis Diagram For each central atom # #
What is the Lewis dot structure for PCl3? - handlebar ... Lewis-dot structure is defined as the structure which represents the number of valence electrons around the atoms. The electrons are represented as dots. Fluorine needs 1 electron to complete its octet. When another fluorine combines, they share 1 electron each forming a single bond. How to Draw Lewis Structure for POCl3 phosphoryl chloride ... How to Draw a Lewis Structure for POCl3? Lewis Structure: : https... PCl3 Lewis Structure, Hybridization, Molecular Geometry ... We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. Another simple formula can also give us the hybridization of PCl3. Lewis structure for POCl3 - CHEMISTRY COMMUNITY Re: Lewis structure for POCl3. P is the central atom because it is the least electronegative in comparison to O and Cl, and it also has the ability to be an expanded octet, which makes the drawing of the lewis structure accurate.
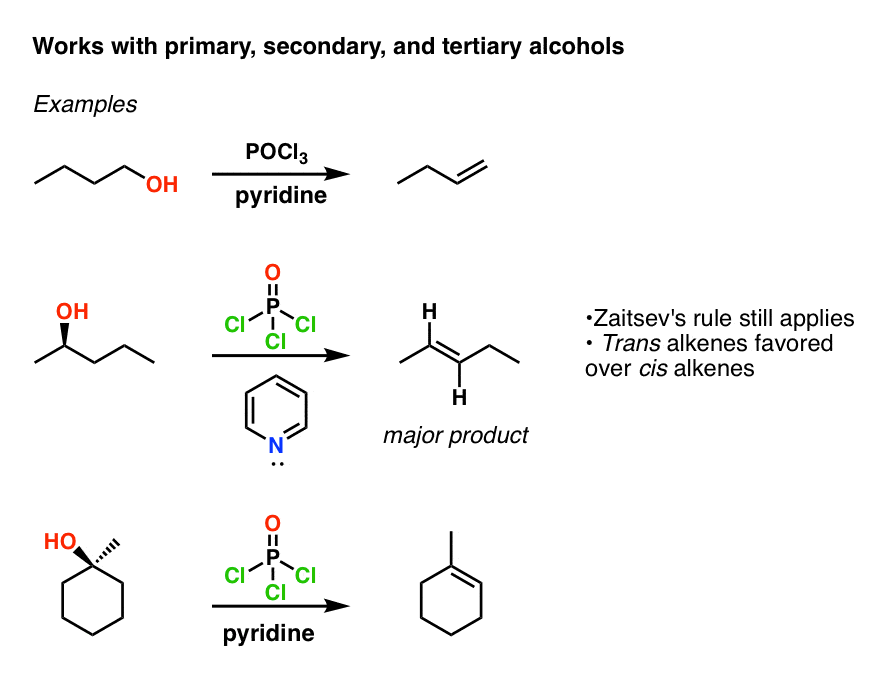
Phosphoryl chloride - Wikipedia Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula P O Cl 3.It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride.It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate
PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.
How to draw PCl3 Lewis Structure? - Science Education and ... Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get ...
POCl3 Lewis Structure, Molecular Geometry, Hybridization ... POCl3 Lewis Structure. The Lewis structure of any molecule helps understand the arrangement of atoms in the molecule, its bond formation, and the valence electrons participating in forming bonds. The valence electrons that take part in forming bonds are called bonding pairs of electrons, whereas those that do not form bonds are called lone ...

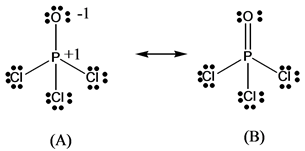



















0 Response to "35 lewis dot diagram for pocl3"
Post a Comment