35 calculating the wavelength of a spectral line from an energy diagram
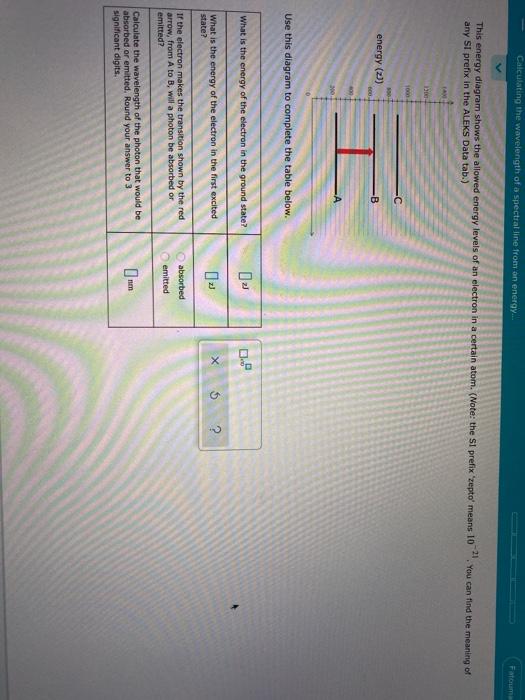
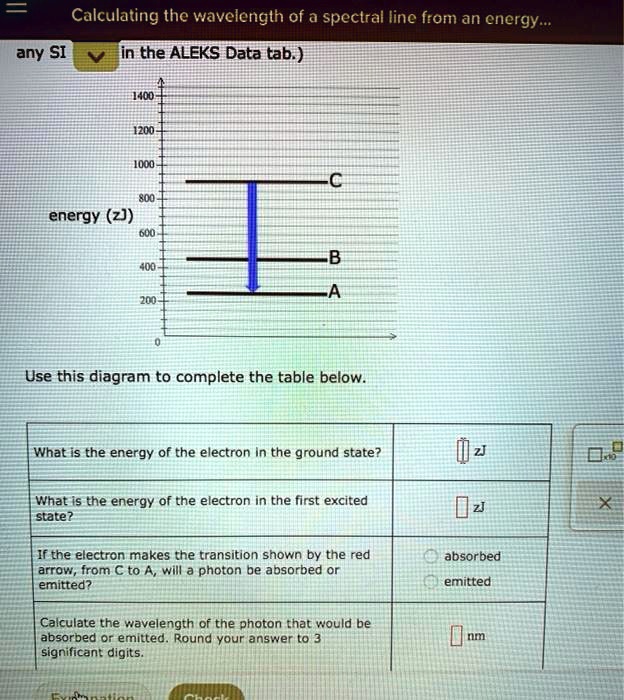
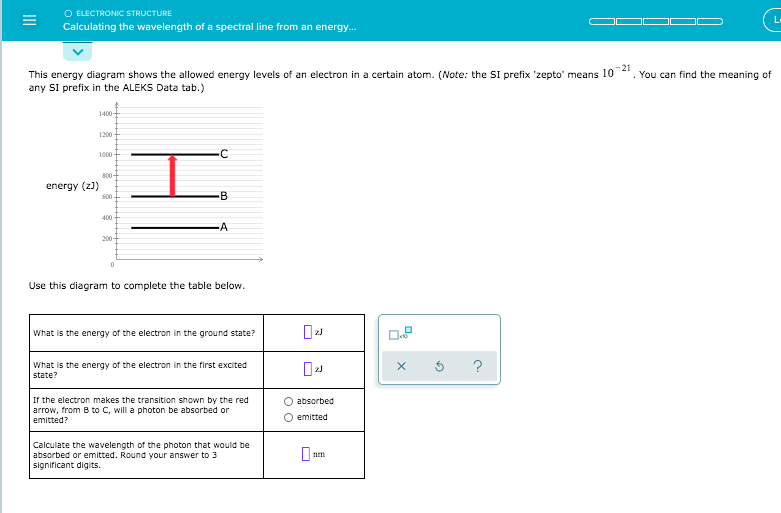
Calculating the wavelength of a spectral line from an energy ... Calculating the wavelength of a spectral line from an energy diagram The lowest possible energy is called the ground state, all other states are called excited states • A has the lowest energy at 350 zJ • B is next at 500 zJ An electron making a transition to a state of lower energy will emit a photon to get rid of excess energy An electron going to a higher state will. Solved O ELECTRONIC STRUCTURE Calculating the wavelength of ... O ELECTRONIC STRUCTURE Calculating the wavelength of a spectral line from an energy... L This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10-21. You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400+ 1200 1000 -C 300- energy (z)) 600 B В 400 -A 200- 0 Use this diagram to.
Energy From Wavelength Example Problem - ThoughtCo In other words, the energy of a photo is directly proportional to its frequency and inversely proportional to its wavelength. All that remains is to plug in the values and get the answer: E = 6.626 x 10 -34 J·s x 3 x 10 8 m/sec/ (633 nm x 10 -9 m/1 nm) E = 1.988 x 10 -25 J·m/6.33 x 10 -7 m E = 3.14 x -19 J. Answer:
Calculating the wavelength of a spectral line from an energy diagram
Calculating Wavelength of a Spectral Line from an Energy Diagram Practice Calculating Wavelength of a Spectral Line from an Energy Diagram with practice problems and explanations. Get instant feedback, extra help and step-by-step explanations. Boost your ... Calculating the wavelenght of a spectral line from an ... To calculate the wavelength of the photon, you'll need to use the relationship between photon energy and wavelength for electromagnetic radiation: 350 zJ 700 zJ = − 700. zJ 350. zJ 350. zJ 350. zJ 12/11/2016 ALEKS In this equation stands for photon energy and for wavelength, is the Planck constant and is speed of light. Calculate the wavelength of the spectral line, when the ... Calculate the wavelength of the spectral line, when the electron in the hydrogen atom undergoes a transition from the energy level 4 to energy level 2.
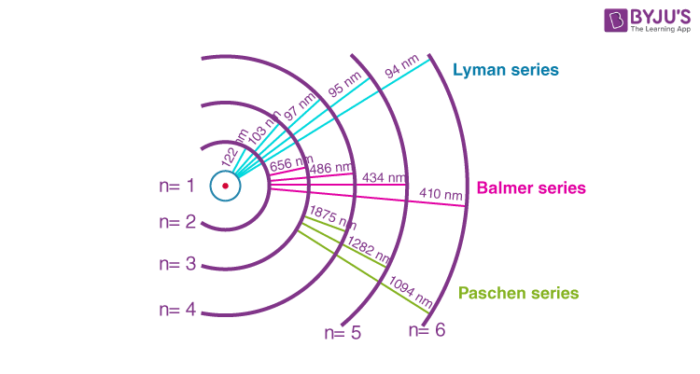
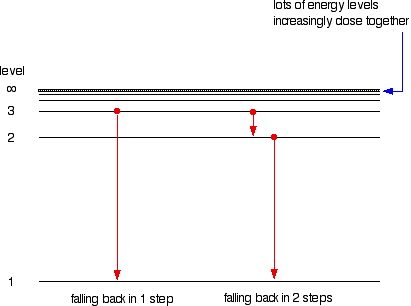
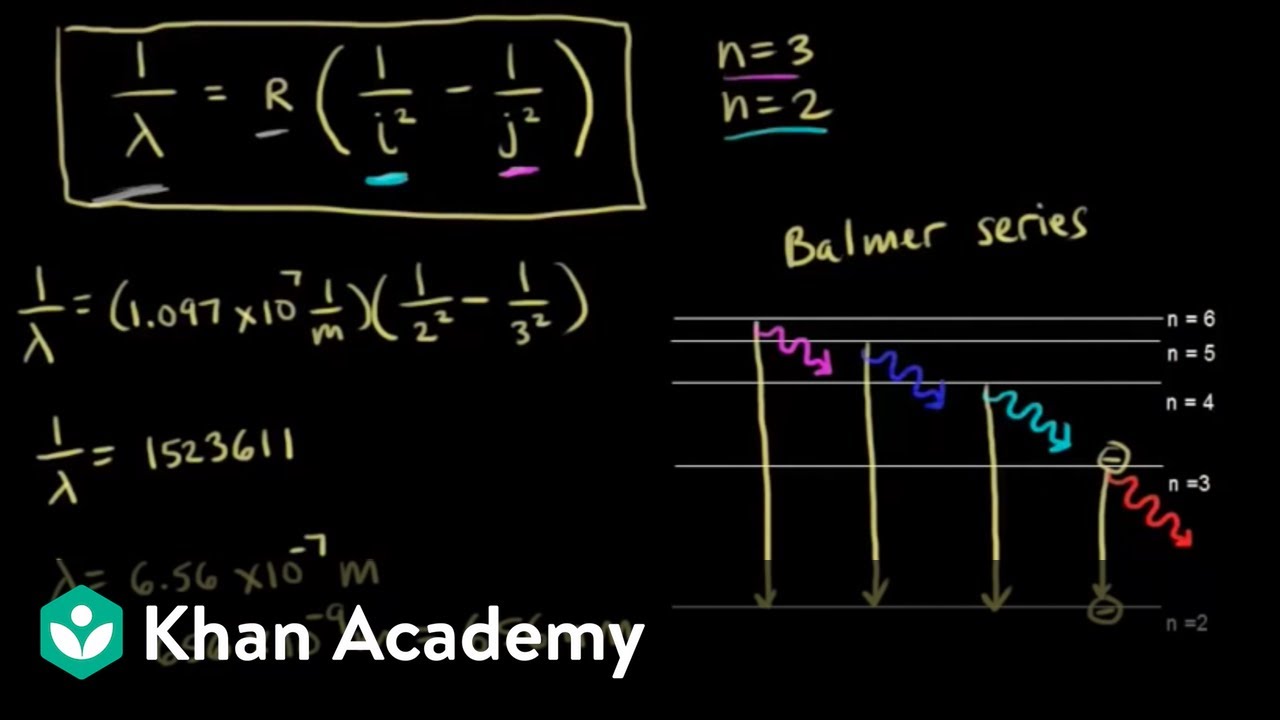
Calculating the wavelength of a spectral line from an energy diagram. Wavelength of a spectral line of energy - 1/14/2017 ALEKS ... Calculating the wavelenght of a spectral line from an energy diagram. University of Toledo. ... 01/14/2017 Electronic Structure Calculating the wavelength of a spectral line from an energy diagram This energy diagram shows the allowed energy levels of an electron in a certain atom. ... Draw the energy level diagram for the line spectra class ... n = 2 from the higher orbits, then the series of spectral lines observed is called the Balmer series. It emits lines of wavelength in the ultraviolet region. Wavelengths in Balmer Series as calculated by Rydberg's formula is- 1 λ = R H ( 1 4 − 1 n 2 2) Note: Calculating Wavelength of a Spectral Line from an Energy Diagram Steps for Calculating Wavelength of a Spectral Line from an Energy Diagram. Step 1: ... How can I calculate the wavelength from energy? | Socratic Wavelength from energy. The formula is. E = hc λ or λ = hc E, where h is Planck's constant. For example, what is the wavelength of a photon that has an energy of. 3.36 × 10⁻¹⁹ J? λ = hc E = 6.626 ×10⁻³⁴J⋅s ×2.998 × 10⁸m⋅s⁻¹ 3.36 × 10⁻¹⁹J = 5.91 × 10⁻⁷ m =. 591 nm.
Solved = O ELECTRONIC STRUCTURE Calculating the wavelength ... What is the energy of the electron in the first excited Х $ ? state? If the electron makes the transition shown by the red arrow, from A to C, Question: = O ELECTRONIC STRUCTURE Calculating the wavelength of a spectral line from an energy... ene b) 600. 100 -B A 200 0 Use this diagram to complete the table below. What is the energy of the ... 8.4d Calculating the wavelength of a spectral line from an ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Spectral Calculator Blackbody Calculator Spectroscopy and remote sensing tools for researchers, teachers, and students Subscribe now for full access to the Spectral Calculator tools. Get priority use of advanced, state-of-the-art radiative transfer algorithms--the same ones used by NASA for many remote sensing missions. Solved Calculating the wavelength of a spectral line from ... Calculating the wavelength of a spectral line from an energy... 1400 1200 С I 1000 800 -B energy 600 400 A 200 Use this diagram to complete the table below. What is the energy of the electron in the ground state? L Х 5 ? What is the energy of the electron in the first excited state?
Solved O ELECTRONIC STRUCTURE Calculating the wavelength of ... Transcribed image text: O ELECTRONIC STRUCTURE Calculating the wavelength of a spectral line from an energy... -21 This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the Si prefix 'zepto' means 10 any Si prefix in the ALEKS Data tab.) . You can find the meaning of 1200 1000 500- energy (2) C B 600 400 200- 0 Use this diagram to complete the table below. Spectral Series- Explained along with Hydrogen spectrum ... The wavelengths of the spectral series is calculated by Rydberg formula. Rydberg formula relates to the energy difference between the various levels of Bohr's model and the wavelengths of absorbed or emitted photons. It is mathematically expressed as-. 1 λ = R Z 2 ( 1 n l 2 − 1 n h 2) Where, 𝜆 is the wavelength. III O ELECTRONIC STRUCTURE Calculating the wavelength ... Transcribed image text: III O ELECTRONIC STRUCTURE Calculating the wavelength of a spectral line from an energy... -21 This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10 any Si prefix in the ALEKS Data tab.) You can find the meaning of 1400 1200 1000 300+ energy (23) C 600 400 B 200 A 0 Use this diagram to complete the ... calculate the wavelength in nanometers of the spectral ... Were number wavelength of spectral lines can be calculated by? Complete step by step answer: The Balmer series wavelengths can be calculated by the formula 1λ=RH [14−1n2], here λ is the wavelength, RH is the Rydberg constant, and n is the level of upper orbit. Also ν=1λ, where ν is the wave number.
ALEKS: Calculating the wavelength of a spectral line from an ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Calculate the wavelength of the spectral line, when the ... Calculate the wavelength of the spectral line, when the electron in the hydrogen atom undergoes a transition from the energy level 4 to energy level 2.
Calculating the wavelenght of a spectral line from an ... To calculate the wavelength of the photon, you'll need to use the relationship between photon energy and wavelength for electromagnetic radiation: 350 zJ 700 zJ = − 700. zJ 350. zJ 350. zJ 350. zJ 12/11/2016 ALEKS In this equation stands for photon energy and for wavelength, is the Planck constant and is speed of light.
Calculating Wavelength of a Spectral Line from an Energy Diagram Practice Calculating Wavelength of a Spectral Line from an Energy Diagram with practice problems and explanations. Get instant feedback, extra help and step-by-step explanations. Boost your ...





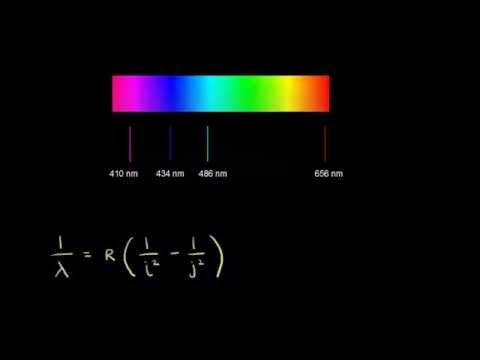






![Expert Verified] Calculate the wavelength, in nanometers, of ...](https://us-static.z-dn.net/files/d6c/d856dde6631aace551a4258108b36e96.png)



0 Response to "35 calculating the wavelength of a spectral line from an energy diagram"
Post a Comment