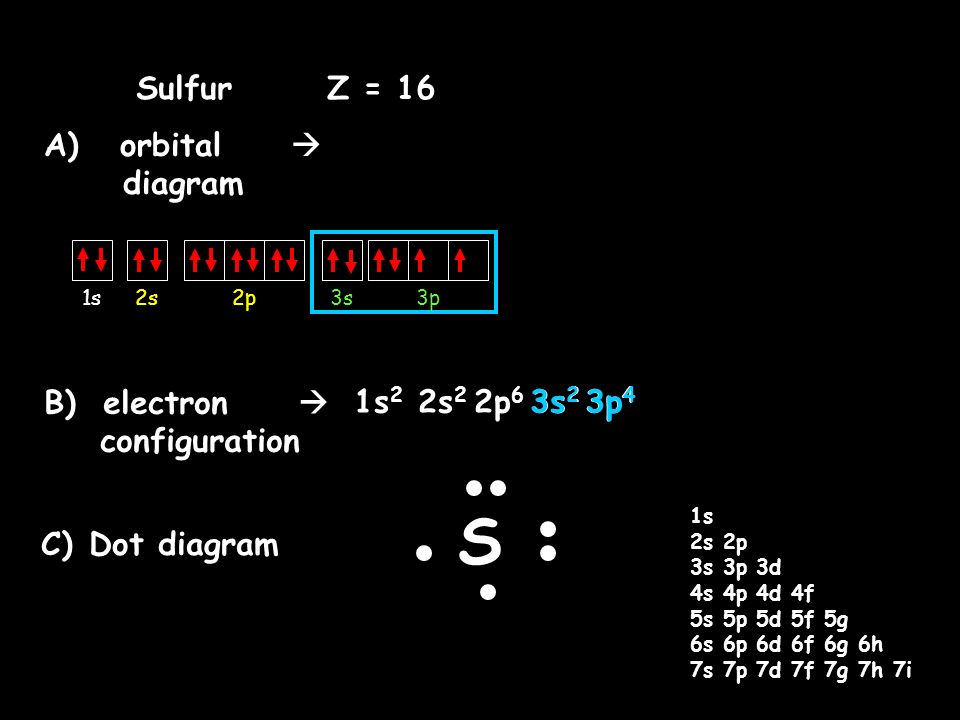
39 orbital filling diagram for sulfur
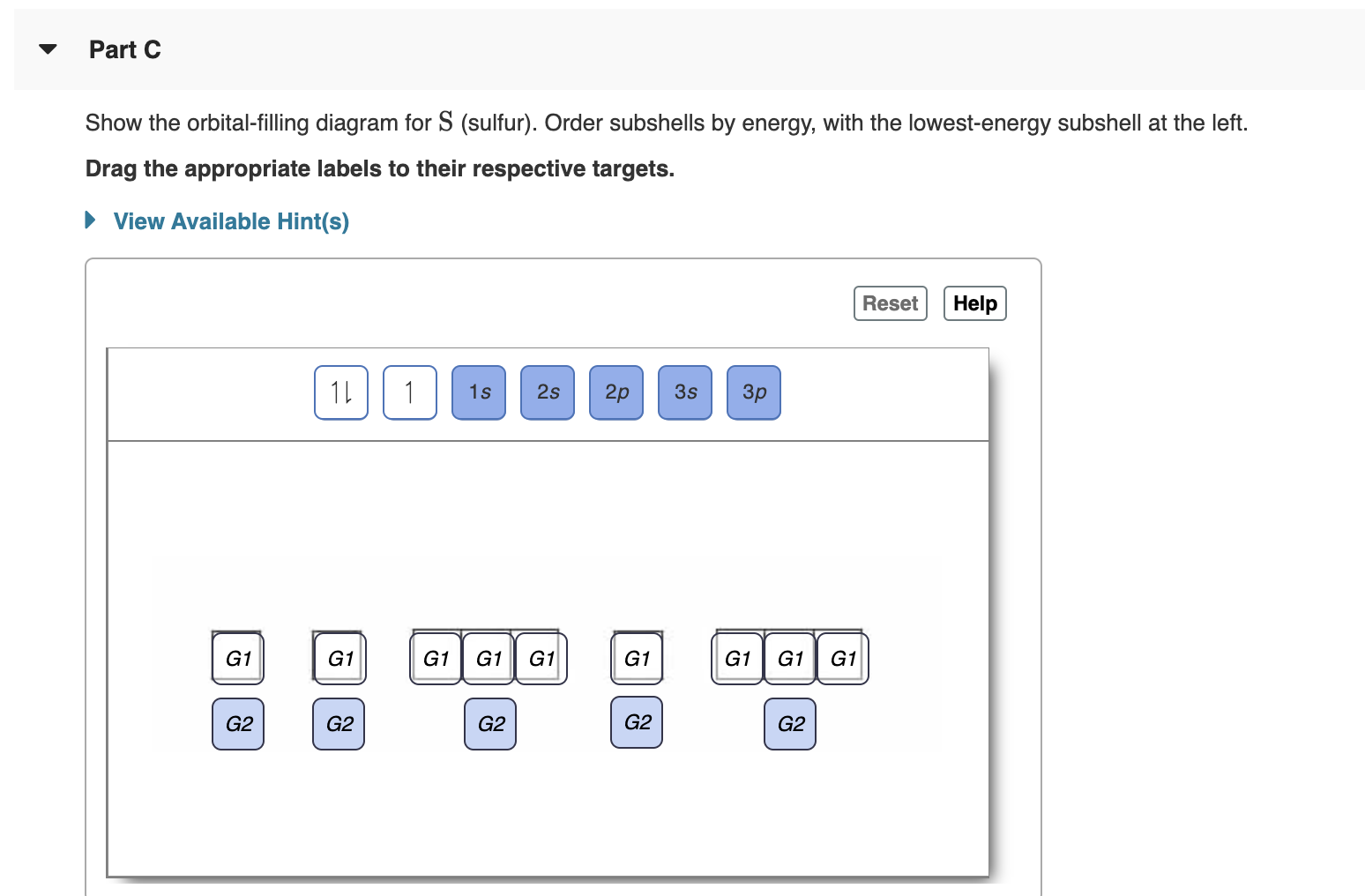
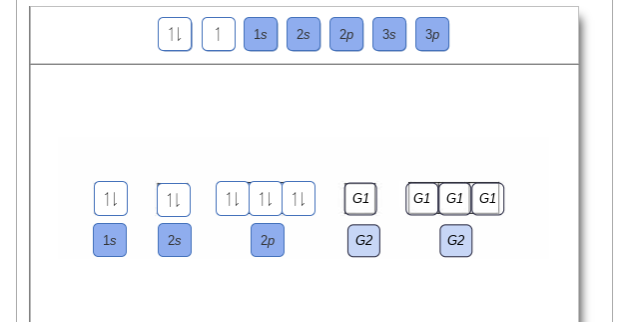
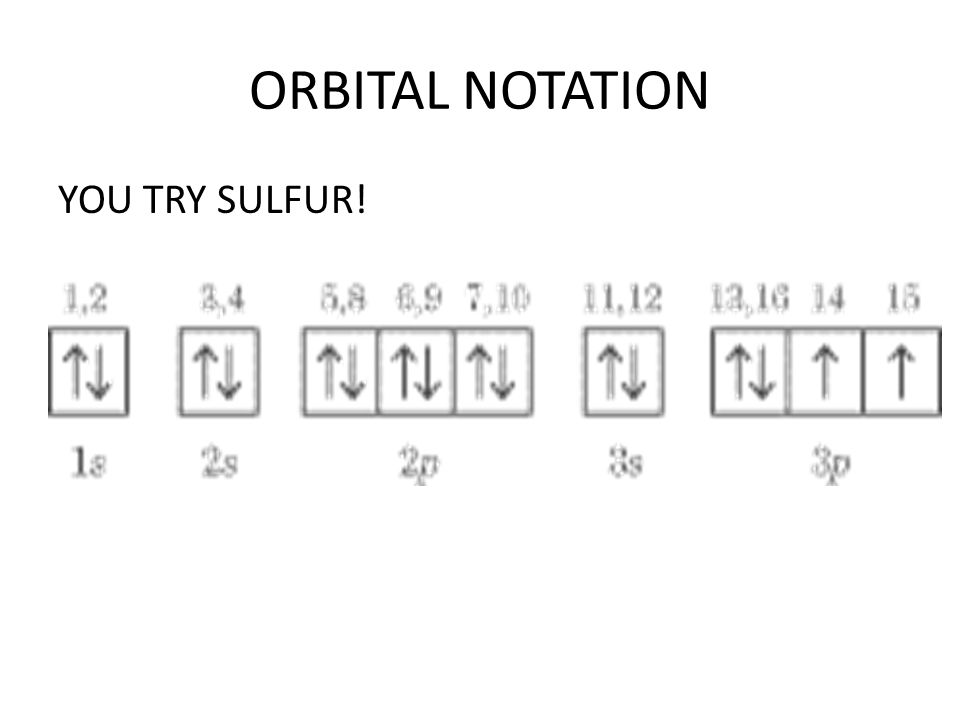
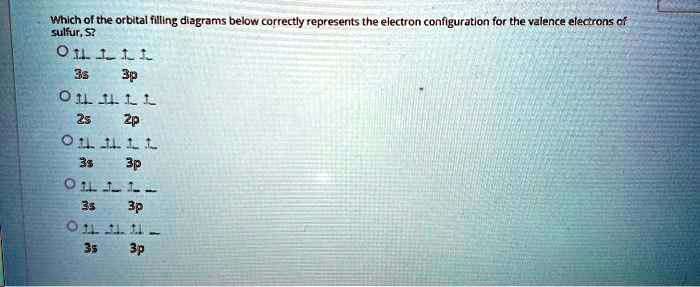
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... Part C. Show the orbital-filling diagram f... | Clutch Prep Part C. Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left To learn to create orbital-filling diagrams. An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy.
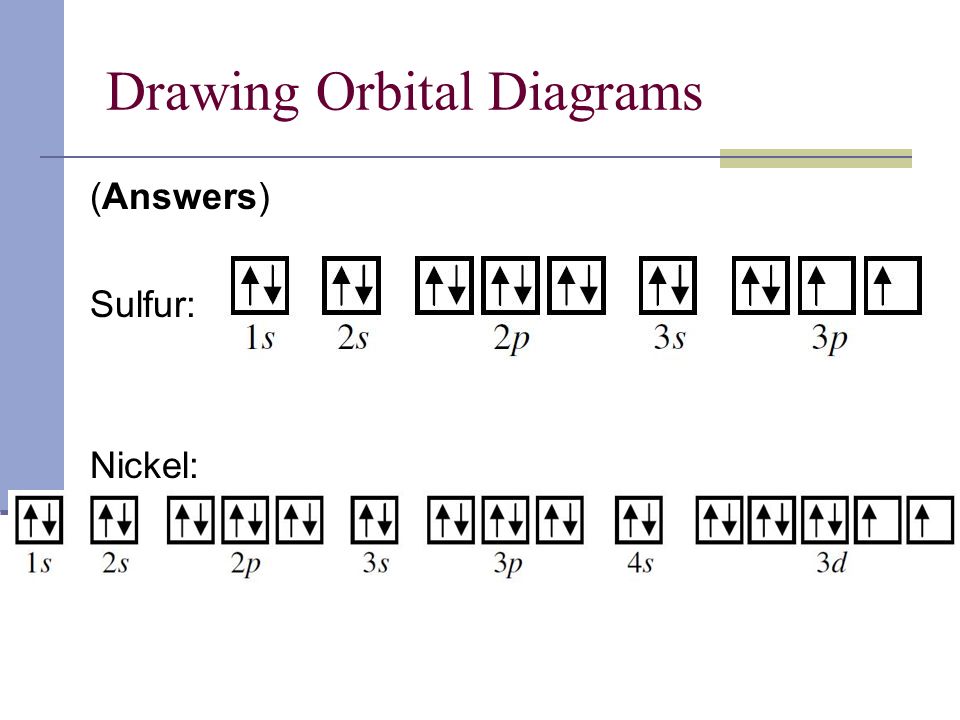
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. ... Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: ... Electron configuration of elements Hund's rule and Orbital filling diagrams. Categories Uncategorized Post navigation. Germanium (Ge) - Periodic Table (Element ...
Orbital filling diagram for sulfur
Sulfur Orbital diagram, Electron configuration, and ... Sulfur is situated in Group 16th or 6A and has an atomic number of 16. The first shell of Sulfur has 2 electrons and the outer shell or valence shell of Sulfur has 6 electrons, hence, the number of valence electrons in the Sulfur atom is 6. The orbital diagram for Sulfur is drawn by following three principles - the Aufbau principle, Hund's ... Show The Orbital-filling Diagram For Br (bromine) Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for . Show the orbital-filling diagram for Br (bromine). Chemistry 2.12: Electron Orbitals Flashcards | Quizlet What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? 2. What is the maximum number electrons that can occupy any d orbital? Use an aufbau diagram. 10. Sulfur has an atomic number of 16. What is the electron configuration for an electronically neutral atom of sulfur?
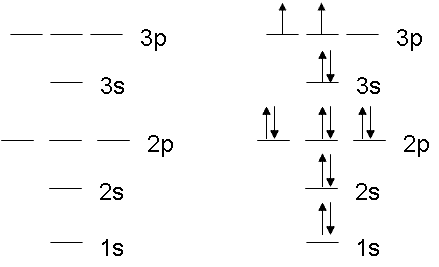
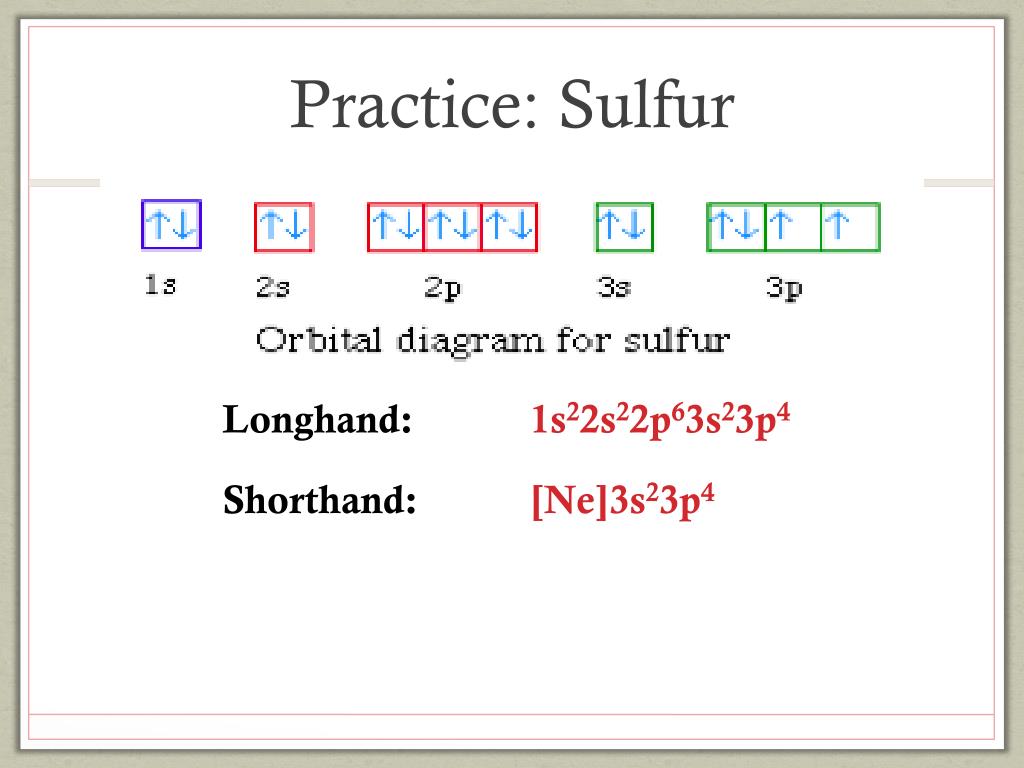
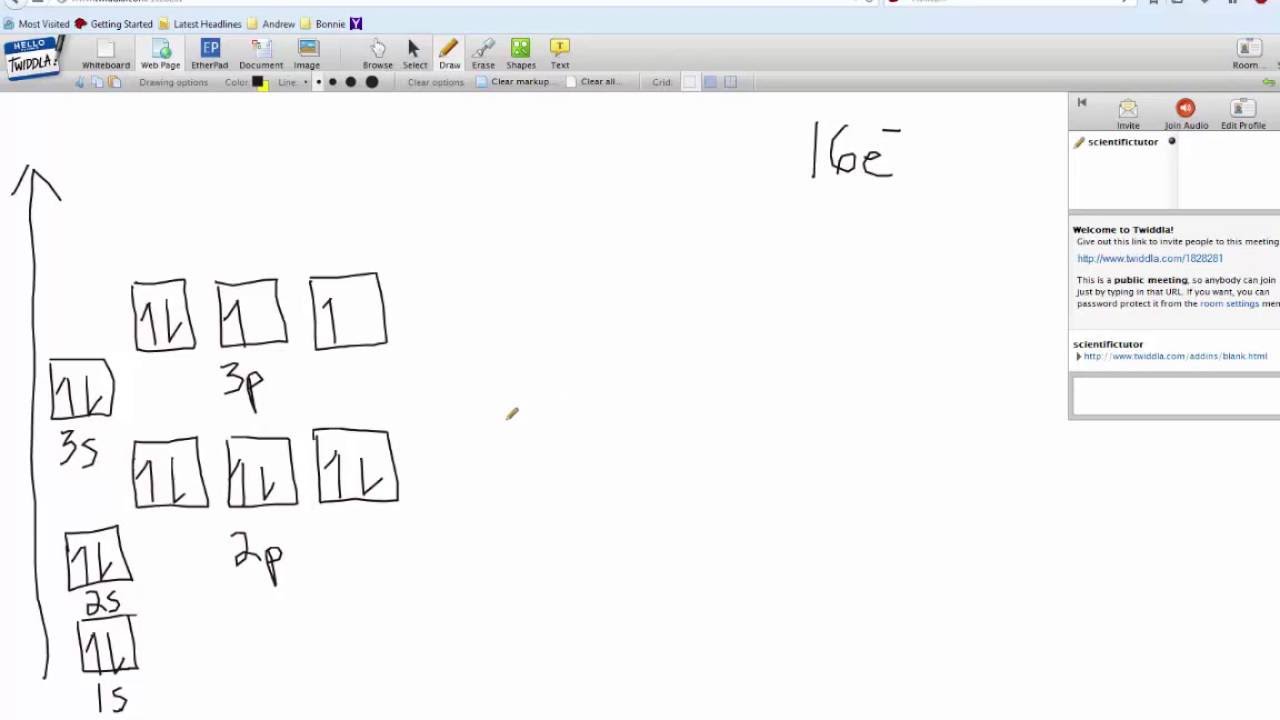
Orbital filling diagram for sulfur. sulfur orbital notation - Pro Hydraulic Sulfur: [Ne]3s²3p⁴ The orbital diagram has nine boxes with two arrows in the first seven and single arrows in the last two. Orbital filling diagram for sulfur. 1s 2 2s 2 2p 6 3p 6. The p, d, and f orbitals have different sublevels. S has 6 valence electrons and needs 2 more to be stable. Solved A Review Constants Periodic Table Learning ... - Chegg An orbital-filling diagram shows the number of electrons in each orbital, which are shown in order of energy. The placement of electrons in orbitals follows a certain set of rules. Part C Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left. PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Show The Orbital Filling Diagram For Sulfur Each arrow represents one electron.Aug 04, · The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals.
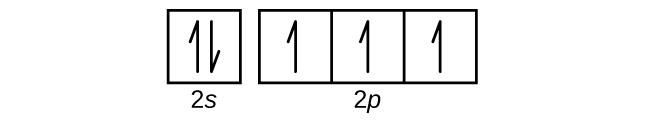
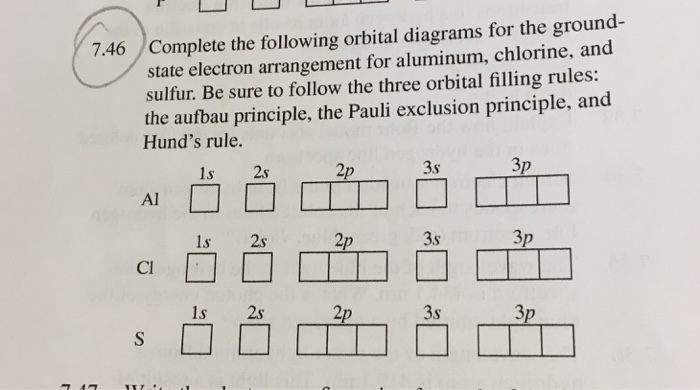
What is the orbital notation for carbon? Carbon has 6 protons and electrons, so it has 2 in the 1S orbital, 2 in the 2S orbital, and 2 in the 1P orbital. This is often expressed as [HE]2S2 2Ps, because it has the same configuration as helium plus 4 additional electrons whose positions are shown after the bracketed element. What Is the Orbital Diagram for Sulfur? - Reference.com What Is the Orbital Diagram for Sulfur? By Staff Writer Last Updated April 03, 2020 The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. Answered: Show the orbital-filling diagram for N… | bartleby Science Chemistry Q&A Library Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 1L 1 1s 2s 2p 3s Зр G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 Part C Show the orbital-filling diagram for S (sulfur). how the orbital-filling diagram for s(sulfur). Stack the ... how the orbital-filling diagram for s (sulfur). Stack the sub shells in order of energy, with the... how the orbital-filling diagram for s (sulfur). Stack the sub shells in order of energy, with the lowest-energy sub shell at the bottom and the highest-energy sub shell at the top. Use the buttons at the top of the tool to add orbitals.
Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Electron Configuration for Sulfur (S) - TerpConnect In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Show The Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Orbital Filling Diagram For Sulfur - schematron.org The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
Solved Show the orbital-filling diagram for S (sulfur ... Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 1 15 2s 2p 3s 3p G1 G1 G1 G1G1 G1 G1 G1 | G1 G2 G2 G2 G2 G2 Submit Part D Show the orbital-filling diagram for Br (bromine).
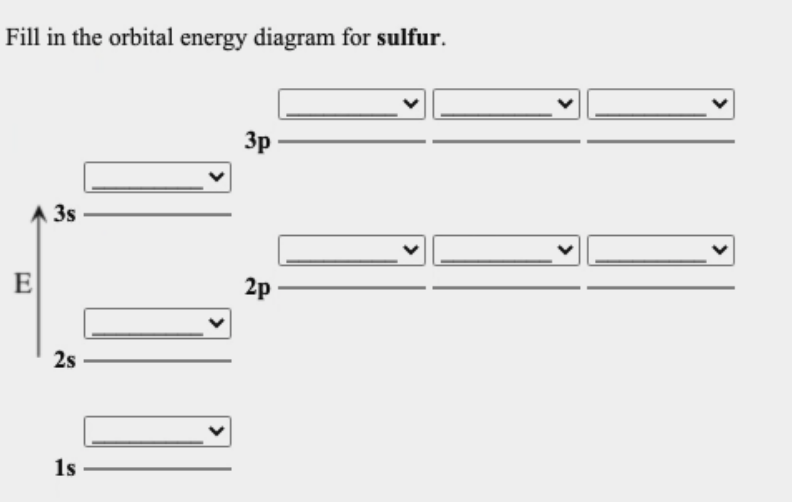
PDF Work on Elctron configuration and orbital diagram 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
Periodic Trends Flashcards - Quizlet Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p⁶3s²3p⁴
What is the orbital diagram for sulfur? - FindAnyAnswer.com In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. Click to see full answer Similarly, you may ask, what is the electron configuration of sulfur? [Ne] 3s² 3p4
Chemistry 2.12: Electron Orbitals Flashcards | Quizlet What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? 2. What is the maximum number electrons that can occupy any d orbital? Use an aufbau diagram. 10. Sulfur has an atomic number of 16. What is the electron configuration for an electronically neutral atom of sulfur?
Show The Orbital-filling Diagram For Br (bromine) Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for . Show the orbital-filling diagram for Br (bromine).
Sulfur Orbital diagram, Electron configuration, and ... Sulfur is situated in Group 16th or 6A and has an atomic number of 16. The first shell of Sulfur has 2 electrons and the outer shell or valence shell of Sulfur has 6 electrons, hence, the number of valence electrons in the Sulfur atom is 6. The orbital diagram for Sulfur is drawn by following three principles - the Aufbau principle, Hund's ...









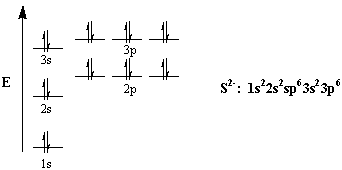



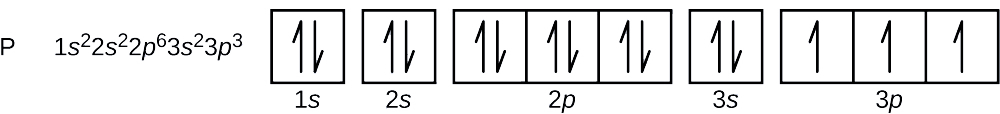




![Electron Configuration | Chemistry [Master]](https://textimgs.s3.amazonaws.com/boundless-chemistry/gram-four-2p-hund-27s-rule.svg)
0 Response to "39 orbital filling diagram for sulfur"
Post a Comment