39 mo diagram for he2
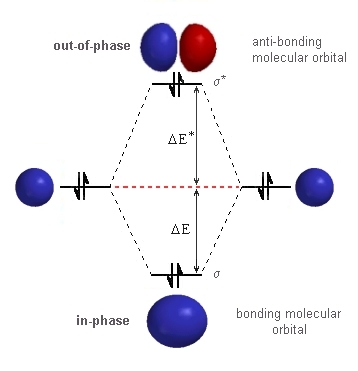
Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each. Therefore, the number of bonding electrons are 2 and the number of anti-bonding electrons are 2. the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.diatomic …
Mo diagram for he2 Mo energy level diagram for he2. Mo diagram for he2+ ion. Mo diagram for he2 2+. Construct an mo diagram for the he2+ ion. 1Molecular Theory Orbital: General Vision of Claro11: 32mins2Molecular Theory Orbital and its Postulates 15: 00mins3LCAO Model, Conditions of OBITALS ATMICAS15: 00mins4Energy Non-viable Diagram for Molecular Orbitals 15: 00mins5Entronic Configuration ...
Mo diagram for he2
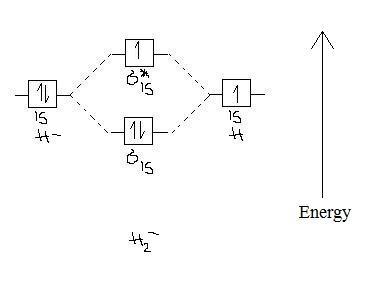
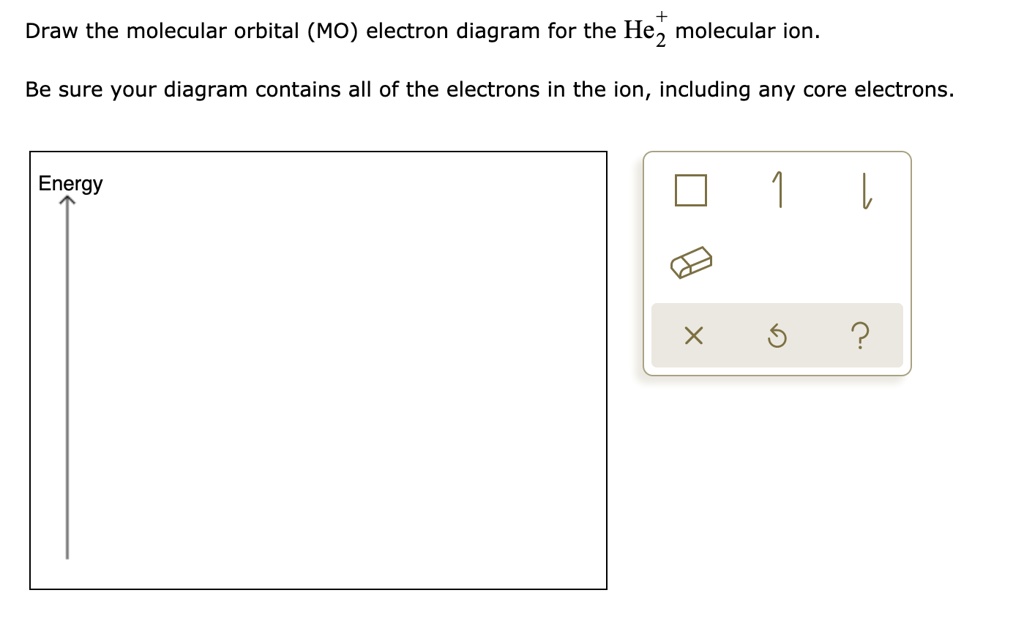
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h2 molecule is shown in figure a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear … Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals. Draw the molecular orbital (MO) electron diagram for the He2^2- molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
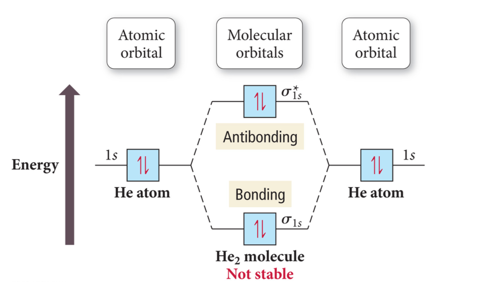
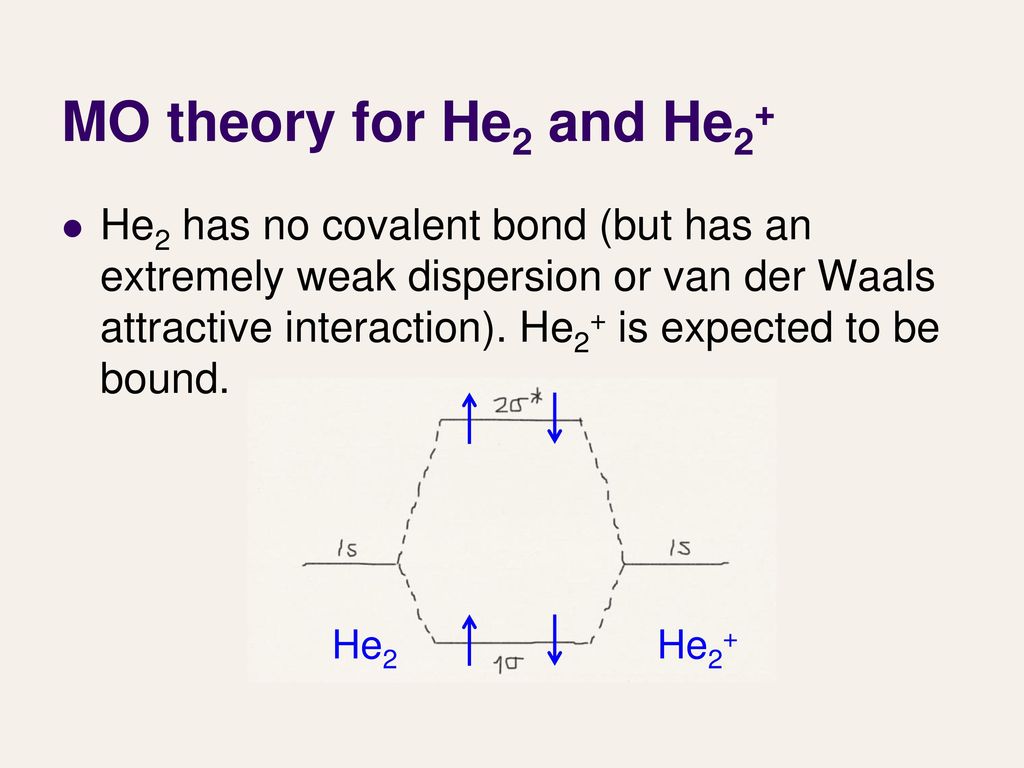
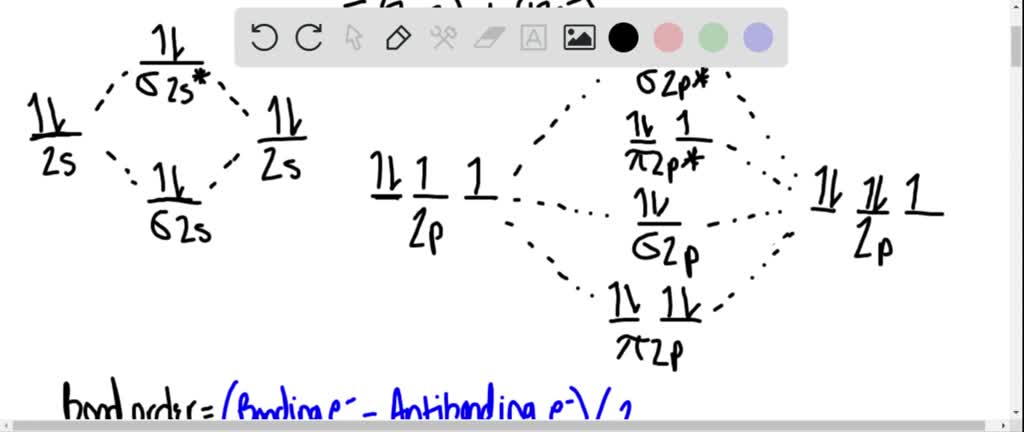
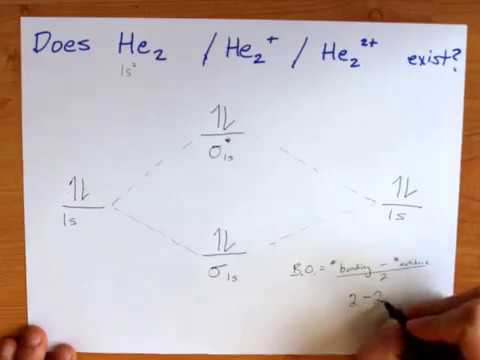
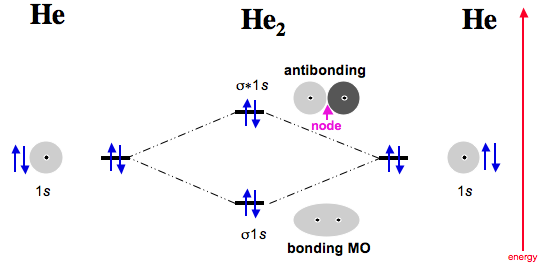
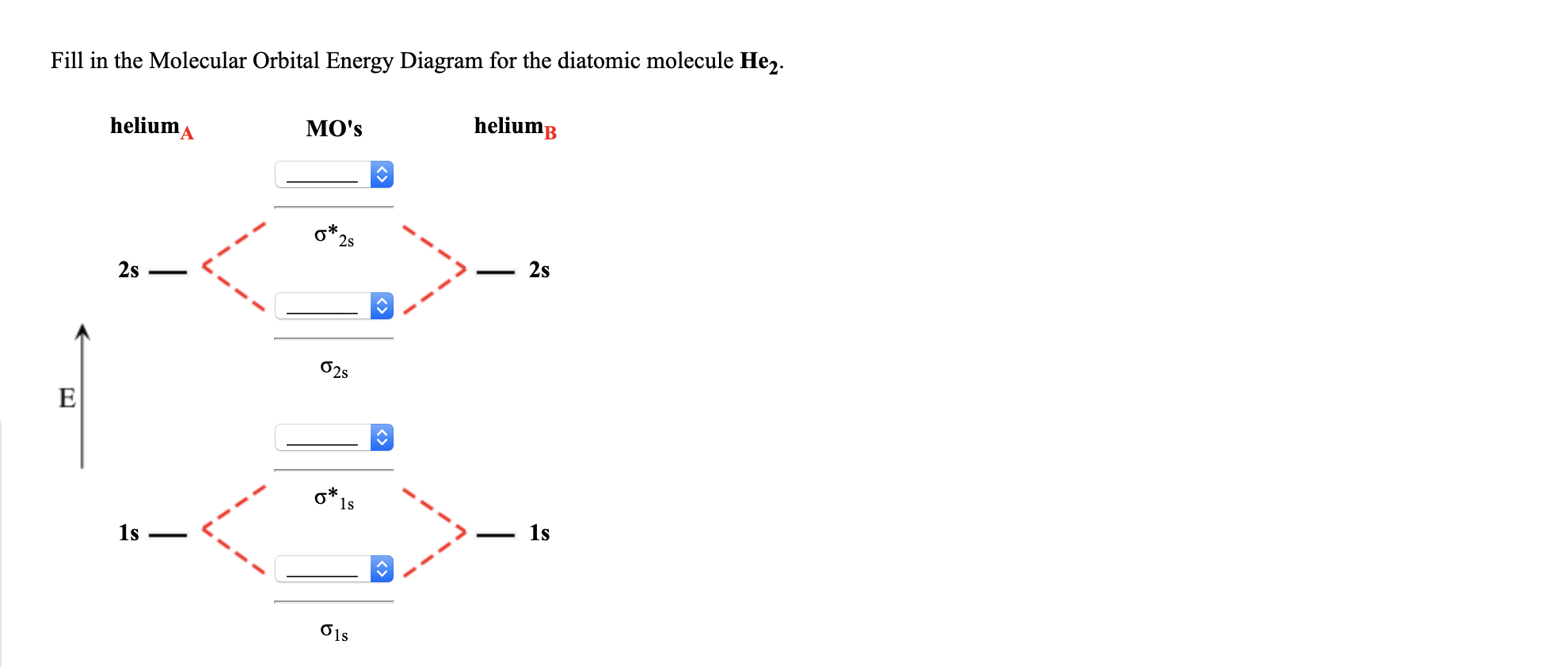
Mo diagram for he2. The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the .... Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding ( 1σ) and .... FREE Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click...1 answer · Top answer: Concepts and reason Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength ... Electronic configuration of Heis 1s2. Molecular Orbital Diagram for He2 is (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)-(No. of electrons in anti-bonding Molecular orbital) =22−2 =0 ∴He2 bond order is 0. There is no bond existing between atoms of He2 . So He2 does not exist. Mo diagram for he2 Construct an mo diagram for the he2+ ion. Mo diagram for he2 2+. Mo diagram for he2+ ion. Mo energy level diagram for he2. Although molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms.
2 (electrons in bonding orbitals - electrons in antibonding orbitals) Draw a complete MO diagram for all the bonds in ethene.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding ... Transcribed image text: Draw the molecular orbital diagram for Hez Drag the appropriate labels to their respective targets. Reset th Atomic orbital Molecular orbitals Atomic orbital 11 ॥ Antibonding ls ls Energy # He atom Bonding Het ion 1+1 11 He2+ ion. Previous question.
B o n d o r d e r = 1 2 [ N b − N a] And we got from the diagram that Helium has two electrons in its bonding molecular orbital and two electrons in its anti-bonding molecular orbital. So its bond order will be: B o n d o r d e r = 1 2 [ 2 − 2] = 0. So, the bond comes out to be zero, therefore the H e 2 molecule is unstable and does not exist. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 . The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding. To answer the question, you must construct a molecular orbital (MO) diagram for the hypothetical He 2 molecule. The σ 1s bonding and antibonding orbitals will be full. Calculating the bond order results in 0. In other words, no bond can be sustained between two He atoms according to MO theory. Top.
Answer (1 of 11): In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4. Molecular Orbitals thus formed are:€1s2€*1s2 It means 2 electrons are in bonding molecular orbitals and 2 are in antibonding molecu...
0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo...
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the...
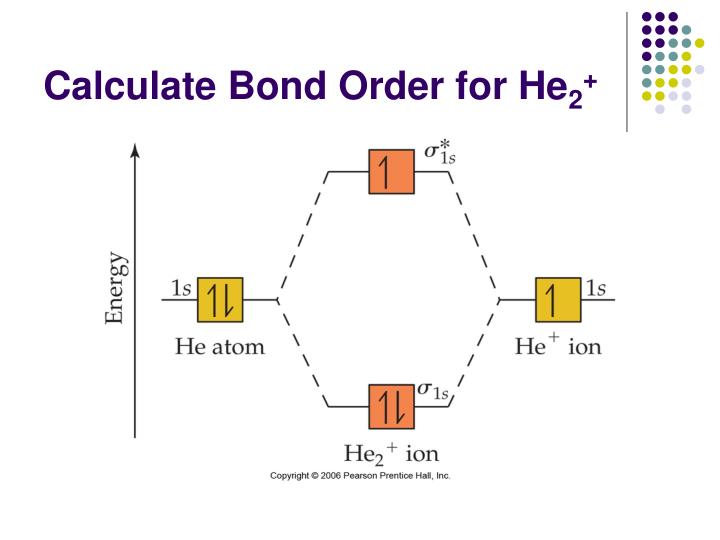
FREE Expert Solution. We're being asked to determine the bond order of He2+. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is:
Draw the molecular orbital (MO) electron diagram for the He2^2- molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h2 molecule is shown in figure a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear …




















0 Response to "39 mo diagram for he2"
Post a Comment