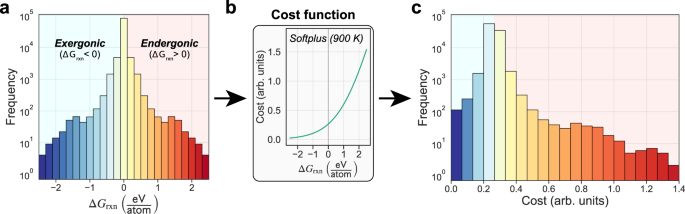
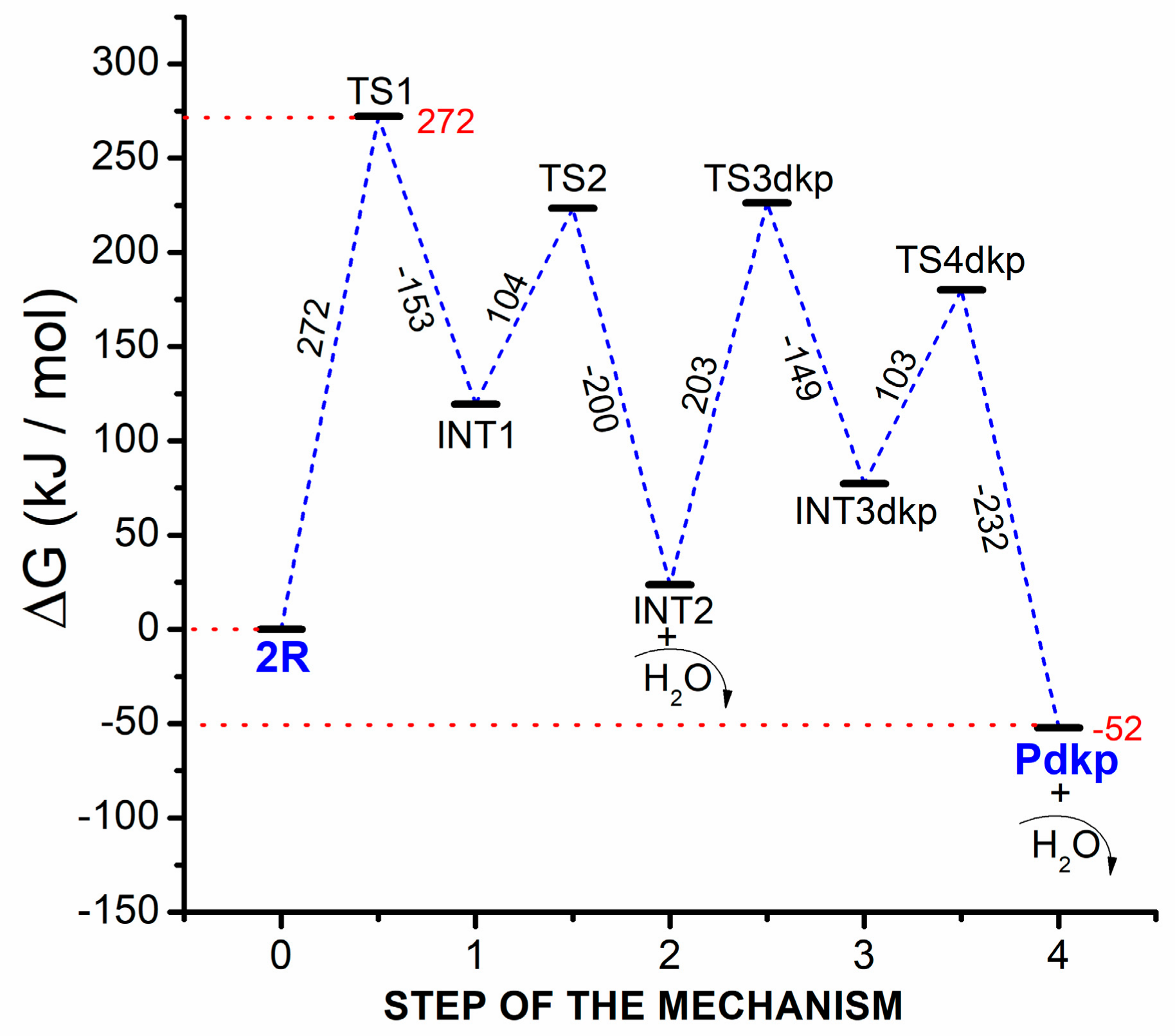
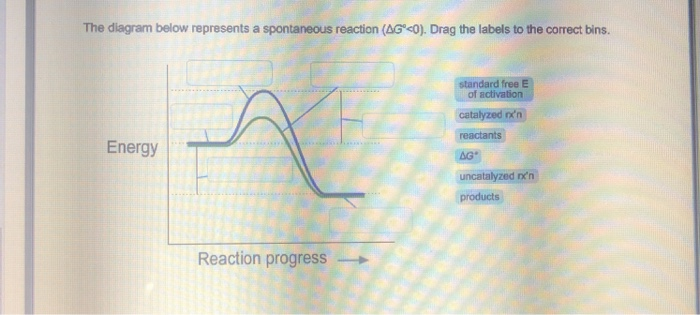
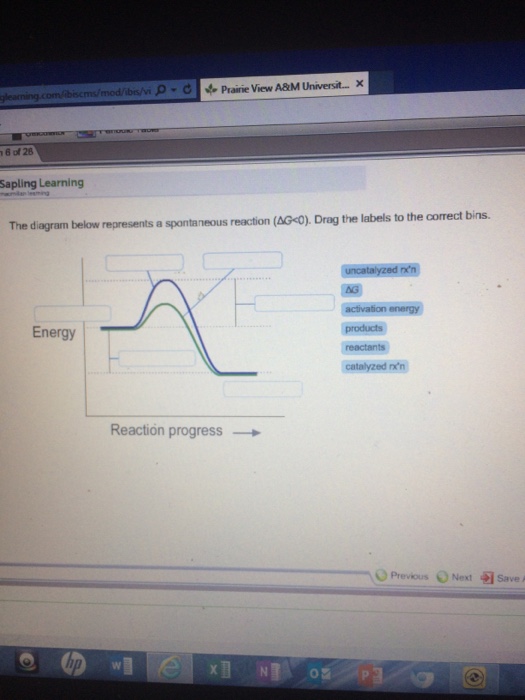
39 the diagram below represents a spontaneous reaction (δg
Difficult math equation that equals 3? (7th grade)? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg° The Kb for methylamine, CH3NH2, at 25°C is ... - Clutch Prep The K b for methylamine, CH 3 NH 2, at 25°C is 4.4 x 10 -4. a. Write the chemical equation for the equilibrium that corresponds to K b. b. By using the value of K b, calculate ΔG° for the equilibrium in part a. c.
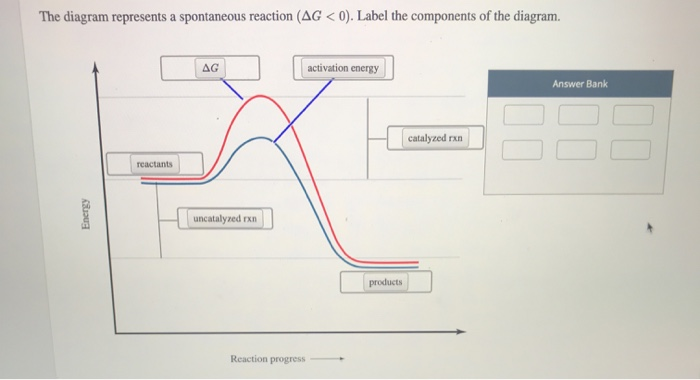
Solution: The diagram below represents a s... | Clutch Prep The diagram below represents a spontaneous reaction (ΔG°<0). Fill in tthe blanks below Learn this topic by watching Gibbs Free Energy Concept Videos
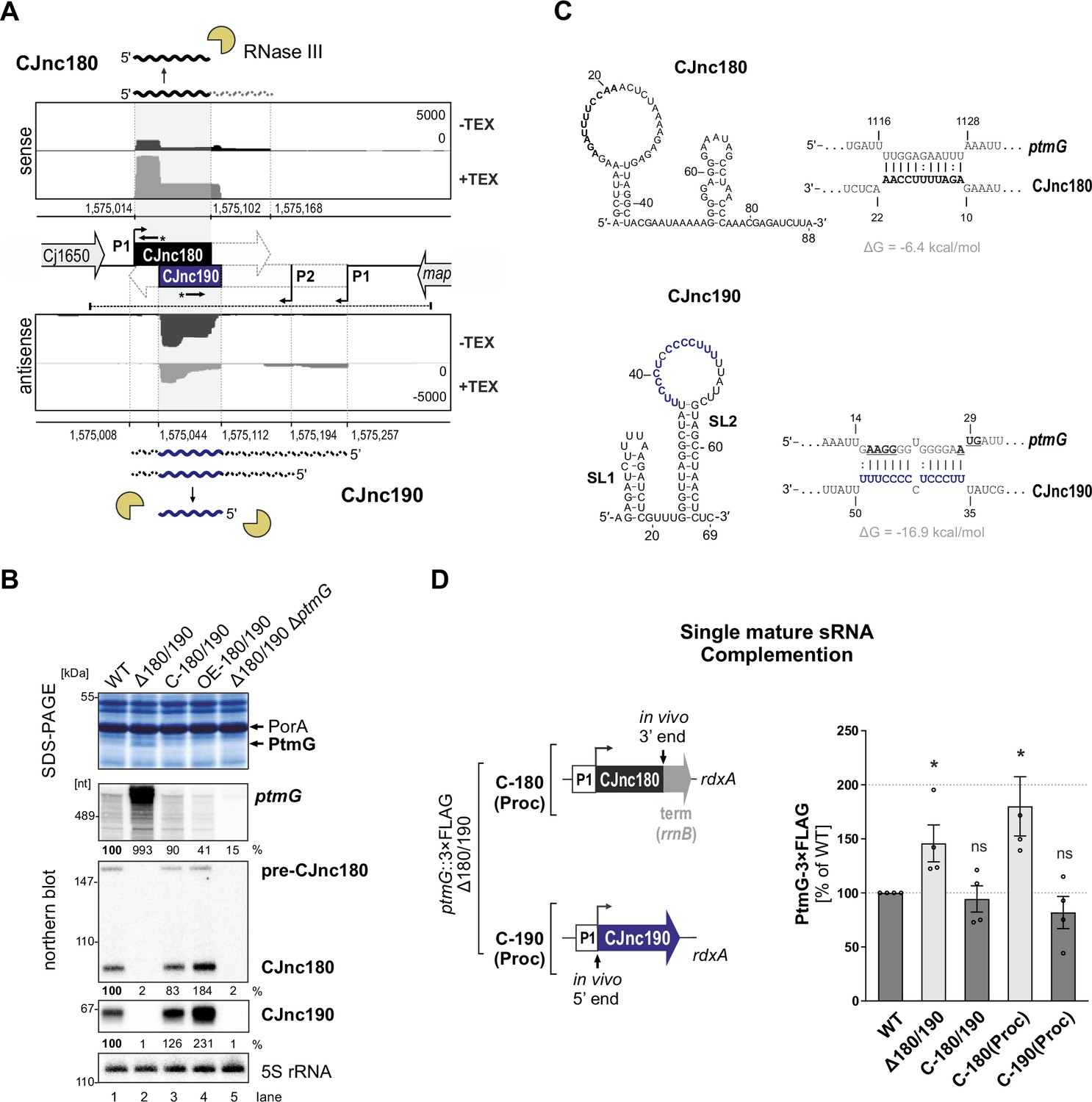
The diagram below represents a spontaneous reaction (δg
The reaction SO2(g) + 2H2S(g) ⇌ 3S(s) + 2H... | Clutch Prep The reaction SO 2 (g) + 2H 2 S(g) ⇌ 3S(s) + 2H 2 O(g) is the basis of a suggested method for removal of SO 2 from power-plant gases. The standard free energy of each substance are ΔG f °S(s) = 0 kJ/mol . ΔG f °H 2 O(g) = -228.57 kJ/mol . ΔG f °SO 2 (g) = -300.4 kJ/mol. ΔG f ° H 2 S (g) = -33.01 kJ/mol. What is the equilibrium constant for the reaction at 298K? In principle, is this ... Solved The diagram represents a spontaneous reaction. Use ... The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? Question: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? sheet how do bulldogs get flat noses? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg°
The diagram below represents a spontaneous reaction (δg. Solved The diagram below represents a spontaneous reaction ... The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n. Question: The diagram below represents a spontaneous reaction (deltaG degree < 0). CHEM 152 UNIT III Flashcards | Quizlet The spontaneous redox reaction in a voltaic cell has _____ A) a negative value of Ecell and a negative value of ΔG. B) a positive value of Ecell and a positive value of ΔG. C) a negative value of Ecell and a positive value of ΔG. D) a positive value of Ecell and a negative value of ΔG. E) a positive value of Ecell and a value of zero for ΔG. Thermodynamics Flashcards - Quizlet For the reaction below ΔG° = + 33.0 kJ, ΔH° = + 92.2 kJ, and ΔS° = + 198.7 J/K. Estimate ... The figure below represents the spontaneous reaction of H2 (shaded spheres) with O2 (unshaded spheres) to produce gaseous H2O. ... According to the diagram above, ΔG° is positive and the equilibrium composition is rich in reactants. CHEM 4311 HW4 *in progress collab Flashcards - Quizlet The reaction SO2(g)+2H2S(g)⇌3S(s)+2H2O(g)SO2(g)+2H2S(g)⇌3S(s)+2H2O(g) is the basis of a suggested method for removal of SO2SO2 from power-plant stack gases. The values below may be helpful when answering questions about the process. Calculate the equilibrium constant KpKpK_p for the reaction at a temperature of 298 KK.
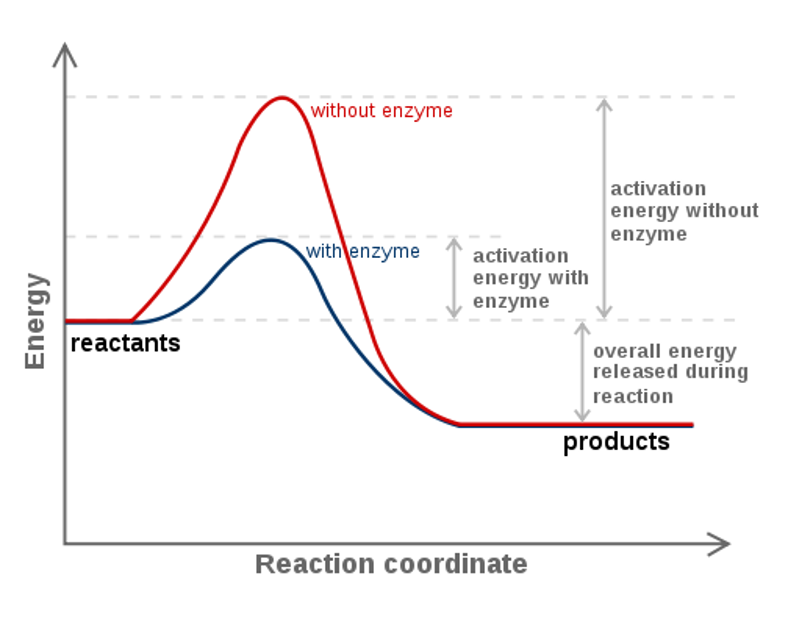
Chem 180 Exam 3 Flashcards - Quizlet The image represents a spontaneous, gaseous reaction at a constant temperature T K. Predict whether ΔH, ΔS, and ΔG for this reaction are positive, negative, or zero. Acetylene, C2H2, can be converted to ethane, C2H6, by a process known as hydrogenation. Solved The diagram represents a spontaneous reaction. Use ... The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? Question: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? Chapter 3 Study Questions Flashcards - Quizlet A chemical reaction is defined as spontaneous if there is a net loss of free energy during the reaction process. However, spontaneous reactions do not always occur rapidly. Favorable biological reactions require _____ to selectively speed up reactions and meet the demands of the cell. a. heat b. ATP c. ions d. enzymes The diagram below represents a spontaneous reaction (δg° Q&A The diagram below represents a spontaneous reaction (δg° Answer General guidance Concepts and reason In energy profile diagram, if the reactants are at lower energy and products are at higher energy, then the reaction is non-spontaneous reaction.
sheet how do bulldogs get flat noses? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg° Solved The diagram represents a spontaneous reaction. Use ... The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? Question: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? The reaction SO2(g) + 2H2S(g) ⇌ 3S(s) + 2H... | Clutch Prep The reaction SO 2 (g) + 2H 2 S(g) ⇌ 3S(s) + 2H 2 O(g) is the basis of a suggested method for removal of SO 2 from power-plant gases. The standard free energy of each substance are ΔG f °S(s) = 0 kJ/mol . ΔG f °H 2 O(g) = -228.57 kJ/mol . ΔG f °SO 2 (g) = -300.4 kJ/mol. ΔG f ° H 2 S (g) = -33.01 kJ/mol. What is the equilibrium constant for the reaction at 298K? In principle, is this ...






:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)






0 Response to "39 the diagram below represents a spontaneous reaction (δg"
Post a Comment