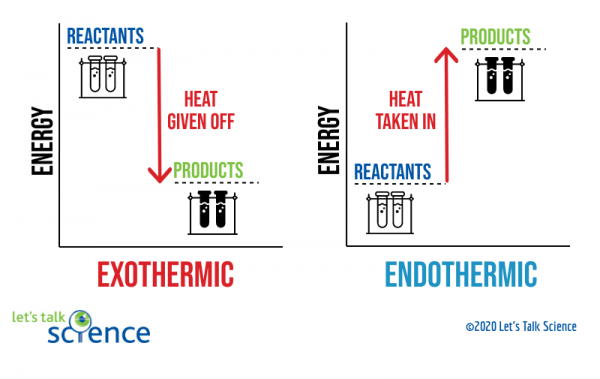
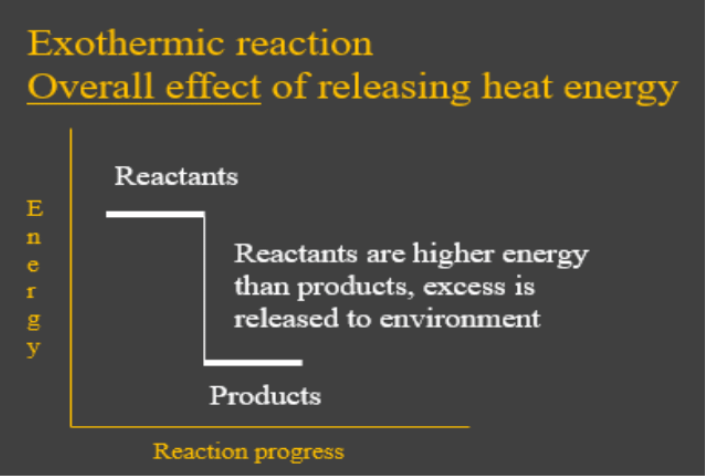
38 this diagram would represent the enthalpy changes in which of the following
PDF Section A - Weebly AS-Level ENERGY CHANGES Q16 Given the following enthalpy changes, What is the standard enthalpy change of formation of iodine trichloride, ICl3(s)? A +176 kJ mol-1 . B -88 kJ mol-1 . C -176 kJ mol -1 . D -214 kJ mol -1 . Q17 Hydrogen peroxide slowly decomposes into water and oxygen. The enthalpy change of PDF ENTHALPY 3. The enthalpy change for the following reaction: H 2 (g) + I 2 (g) 2HI(g) is -9 kJmol-1 Use this information and the values in the above table to calculate the bond dissociation energy for the H-I bond. 4. Explain why using bond dissociation energies only give you an approximate value for the overall energy change Measuring enthalpy changes
How to Draw & Label Enthalpy Diagrams - Video & Lesson ... For the following reaction: 2H_2 (g) + O_2 (g) to 2H_2O (g) Delta H = -483.6 kJ Draw the enthalpy diagram, and label the activation energy. Create an account to start this course today
This diagram would represent the enthalpy changes in which of the following
PDF IB Topics 6 & 16 Multiple Choice Practice - Weebly The diagram shows the energy profile for a catalysed and uncatalysed reaction. Which represents the enthalpy change, ΔH, and the activation energy, E a, for the catalysed reaction? 2. Excess magnesium powder was added to a beaker containing hydrochloric acid, HCl (aq). Hess's Law and enthalpy change calculations Enthalpy change is simply a particular measure of energy change. You will remember that the enthalpy change is the heat evolved or absorbed during a reaction happening at constant pressure. I have labelled the vertical scale on this particular diagram as enthalpy rather than energy, because we are specifically thinking about enthalpy changes. Will mark as brainliest. this diagram would represent the ... In the diagram shown above, abcd is a parallelogram. the ratio of the area of triangle agb to the area of triangle cge is 9: 25. if cg=10 and ge=15 find ag. Answers Mathematics, 25.06.2019 17:10
This diagram would represent the enthalpy changes in which of the following. AP Chemistry: Midterm Review Flashcards - Quizlet Based on the information in the table, which of the following mathematical expressions is correct for the estimated enthalpy change for the reaction? Reaction 1:N2O4(g)→2NO2(g) ΔH1=+57.9kJ Based on the information for two different reactions given above, which of the following gives the quantities needed to calculate the enthalpy change for ... Arrows in the energy diagram below represent enthalpy ... Iron oxide reacts with aluminum in an exothermic reaction. Fe2O3(s) + 2Al(s) -> 2Fe(s) + Al2O3(s) The reaction of 5.00 g Fe2O3 with excess Al evolves 26.6 kJ of energy in the form of heat. Calculate the enthalpy change per mole of Fe2O3 reacted Study 16 Terms | sem 2 chem 4.06 Flashcards - Quizlet There are two key points about enthalpy that are related to chemistry: - Most chemical reactions occur at constant pressure and volume, so in chemistry—and in this course—we will refer to enthalpy as it relates to the thermal energy of the system (not work). 4.06 Changes in Enthalpy Quiz Flashcards | Quizlet Start studying 4.06 Changes in Enthalpy Quiz. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
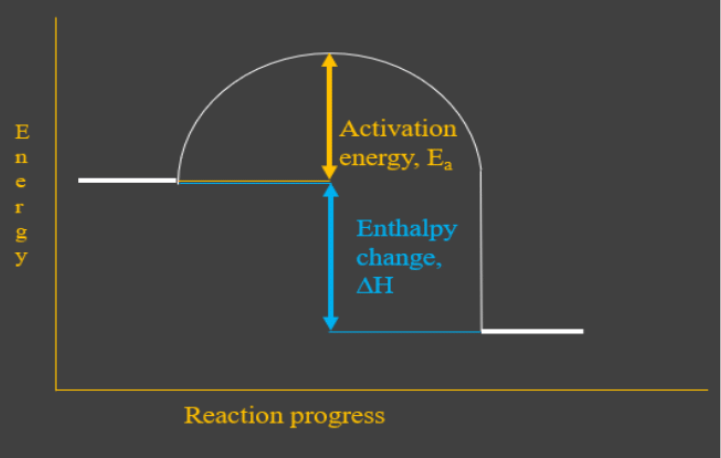
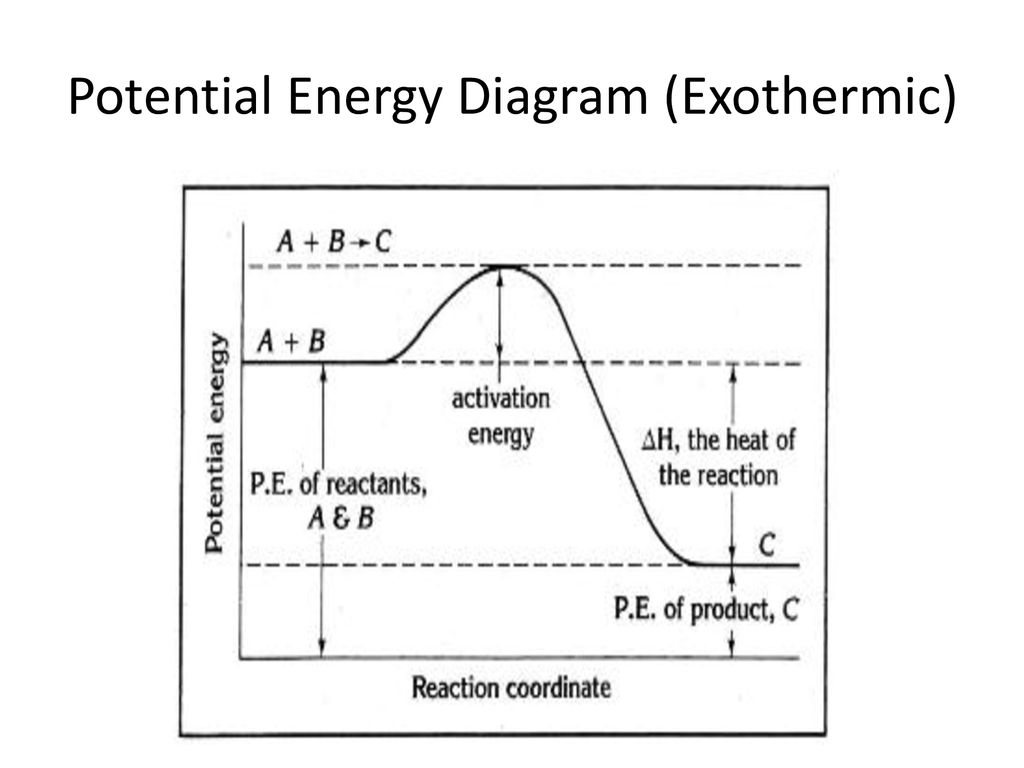
PDF Enthalpy diagram & energy stoichiometry Potential Energy diagrams and Energy Stoichiometry Use the diagram to answer questions 1-5. .Which letter represents the activation energy for the reaction? 2.Which letter represents the enthalpy change for the reaction? 3.1s the reaction in the diagram exothermic or endothermic? 4.1s the enthalpy change positive or negative? AP CHEM UNIT 7 MCQ Flashcards | Quizlet The particle diagram shown above represents the dissolution of CuCl(s) assuming an equilibrium concentration for Cu+ ions of about 4×10−4M in a saturated solution at 25°C. The equilibrium being represented is shown in the following chemical equation. Represent the potential energy/enthalpy change in the ... Represent the potential energy/enthalpy change in the following processes graphically. (a) Throwing a stone from the ground to roof. (b) 1/2 H 2 (g) + 1/2 Cl 2 (g) ⇄ HCl(g) Δ r H Θ = -92.32 kJ mol -1 . In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity? Solved The following Lewis diagram represents the valence ... The following Lewis diagram represents the valence electron configuration of a main-group element. (Enter the answer using superscript.) If this element is in period 4, its valence electron configuration is pt Using average bond enthalpies (linked above), estimate the enthalpy change for the following reaction: 2HCl (g) + Brz (9) >2HBr (g ...
This diagram would represent the enthalpy changes in which ... This diagram would represent the enthalpy changes in which of the following? - 9007542 bmisses bmisses 03/03/2018 Chemistry College answered This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid 1 See answer bmisses is waiting for your help. Add your answer and earn points. The diagram illustrates the enthalpy changes of a set of ... Click here👆to get an answer to your question ️ The diagram illustrates the enthalpy changes of a set of reactions.Which statements are correct? 1 . The enthalpy change for the transformation U → R is + 42 kJ mol^-1 2 . The enthalpy change for the transformation T → S is endothermic. 3 . The enthalpy change for the transformation R → T is - 33 kJ mol^-1 . Solved Which of the following statements concerning ... Transcribed image text: Which of the following statements concerning potential energy diagrams is true? a) The activation energy of a reaction is proportional to its enthalpy change b) The activation energy is measured from the origin to the top of the peak c) The activation energy of the reverse reaction may be calculated from the diagram d) The peak on this diagram represent an ineffective ... Quiz & Worksheet - Drawing & Labeling Enthalpy Diagrams ... How to Draw & Label Enthalpy Diagrams - Quiz & Worksheet. Choose an answer and hit 'next'. You will receive your score and answers at the end. Glucose + Oxygen goes to carbon dioxide and water ...
Which of the following do(es) not represent Δ H formation ... The enthalpy change for the following process at 2 5 o C and under constant pressure at 1 atm are as follows: C H 4 (g) C (g) + 4 H (g) Δ r H = 3 9 6 k c a l / m o l e C 2 H 6 (g) 2 C (g) + 6 H (g) Δ r H = 6 7 6 k c a l / m o l e
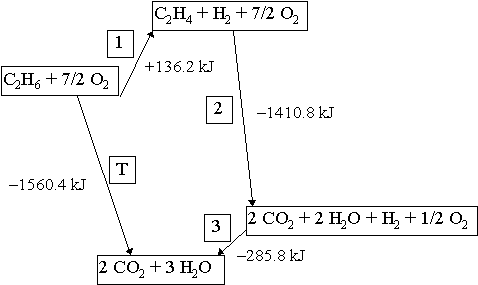
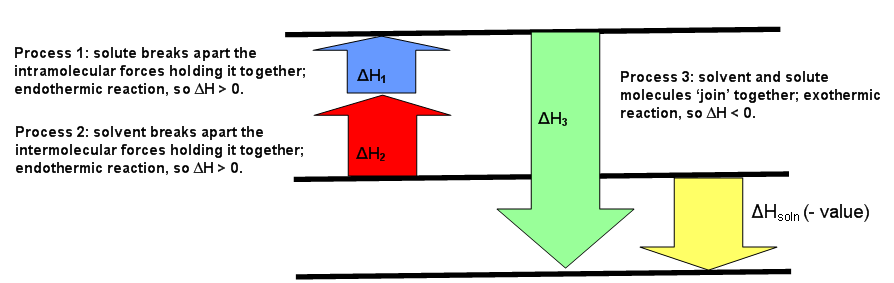
Energetics: 4.32 - Hess' law energy cycles and diagrams The enthalpy level diagram can now be constructed. In this case, the red dotted line is obtained by subtracting the small blue dotted line from the longer blue dotted line. That is, the enthalpy of 4 - 2 = -463 - (-124) = -339 kJ. Therefore the standard enthalpy of formation, ΔH f, of phosphorus(III) chloride = -339 kJ
The given enthalpy diagram represents which of the following 3. A reversible cyclic process for an ideal gas is shown below. Here, P, V, and T are pressure, volume and temperature, respectively. The thermodynamic parameters q, w, H and U are heat, work, enthalpy and internal energy, respectively.
PDF Representing a Reaction with a Potential Energy Diagram Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
DOCX GitHub Pages The diagram shows the distribution of energy for the molecules in a sample of gas at a given temperature, T1 (a) In the diagram . E. a. represents the . activation energy . for a reaction. Define this term. (b) On the diagram above draw another curve to show the energy distribution for the same gas at a higher temperature. Label the curve T. 2. (c)
five marks Use the following diagram to answer the ... 3. (five marks) Use the following diagram to answer the questions below. Briefly summarize what each label on the diagram represents. Also, describe how you can tell if this diagram represents an exothermic or endothermic reaction. A = Ea forward B = enthalpy change C = Ea reverse Reaction is endothermic as potential energy of products i.e. forward reaction is greater than the reactant.
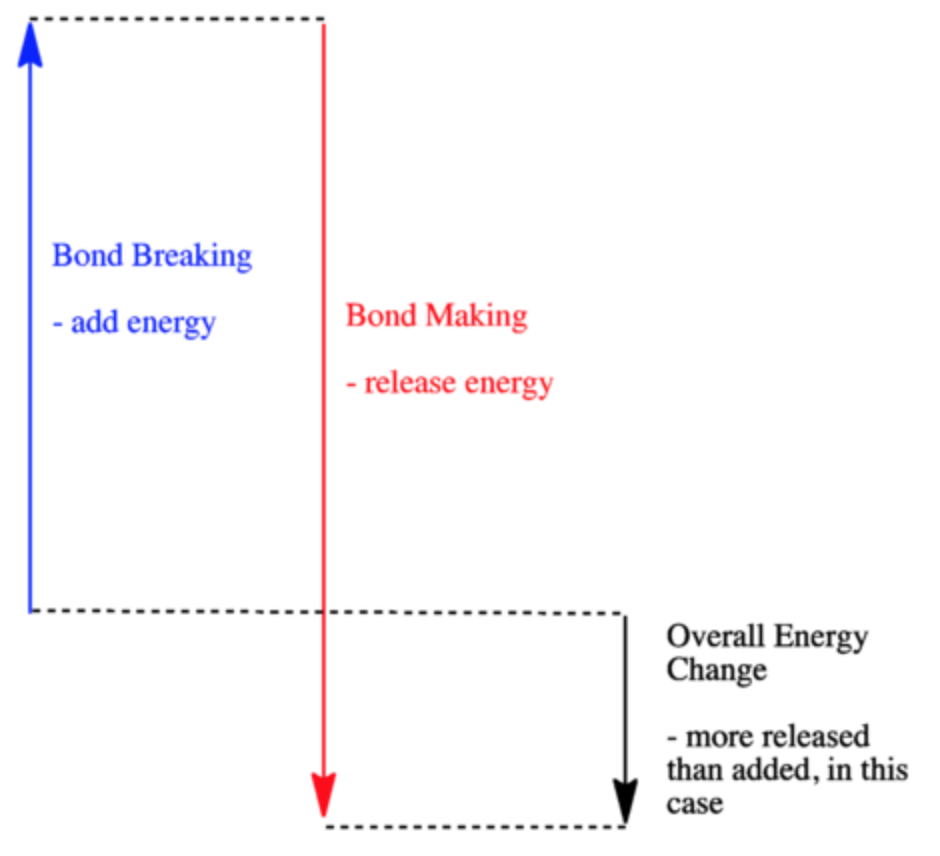
Solved 9. a. If ∆H1 represents enthalpy for bond breaking ... a. If ∆H1 represents enthalpy for bond breaking and ∆H2 represents enthalpy for bond formation write down the mathematical equation for determining enthalpy of reaction. Draw an enthalpy diagram that will correctly represent the enthalpy of reaction using bond. enthalpy. b. Calculate the enthalpy change of the following reaction from the ...
Thermodynamics Conceptual | Chemistry Quiz - Quizizz Q. CO (g) + 2 H 2(g) ↔ CH 3 OH (g) K = 2.2 x10 4 at 298 K. A stoichiometric mixture of CO (g) and H 2(g) was allowed to react in two different rigid containers at a constant temperature of 298 K. The reaction is represented by the equation above. Diagram 1 represents the uncatalyzed reaction and diagram 2 represents the catalyzed reaction one hour after the reactants were mixed.
Chemistry 4.06 Quiz: Changes in Enthalpy Cheat Sheet ... This diagram would represent the enthalpy change in which of the following? liquid water freezing A 10 g gold coin is heated from 25°C to 50°C (CAu is 0.13 J/g-°C).
Will mark as brainliest. this diagram would represent the ... In the diagram shown above, abcd is a parallelogram. the ratio of the area of triangle agb to the area of triangle cge is 9: 25. if cg=10 and ge=15 find ag. Answers Mathematics, 25.06.2019 17:10
Hess's Law and enthalpy change calculations Enthalpy change is simply a particular measure of energy change. You will remember that the enthalpy change is the heat evolved or absorbed during a reaction happening at constant pressure. I have labelled the vertical scale on this particular diagram as enthalpy rather than energy, because we are specifically thinking about enthalpy changes.
PDF IB Topics 6 & 16 Multiple Choice Practice - Weebly The diagram shows the energy profile for a catalysed and uncatalysed reaction. Which represents the enthalpy change, ΔH, and the activation energy, E a, for the catalysed reaction? 2. Excess magnesium powder was added to a beaker containing hydrochloric acid, HCl (aq).

:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)

















0 Response to "38 this diagram would represent the enthalpy changes in which of the following"
Post a Comment