38 energy diagram endothermic and exothermic reaction
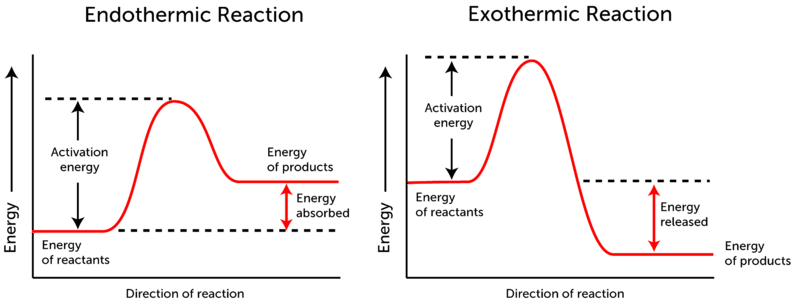
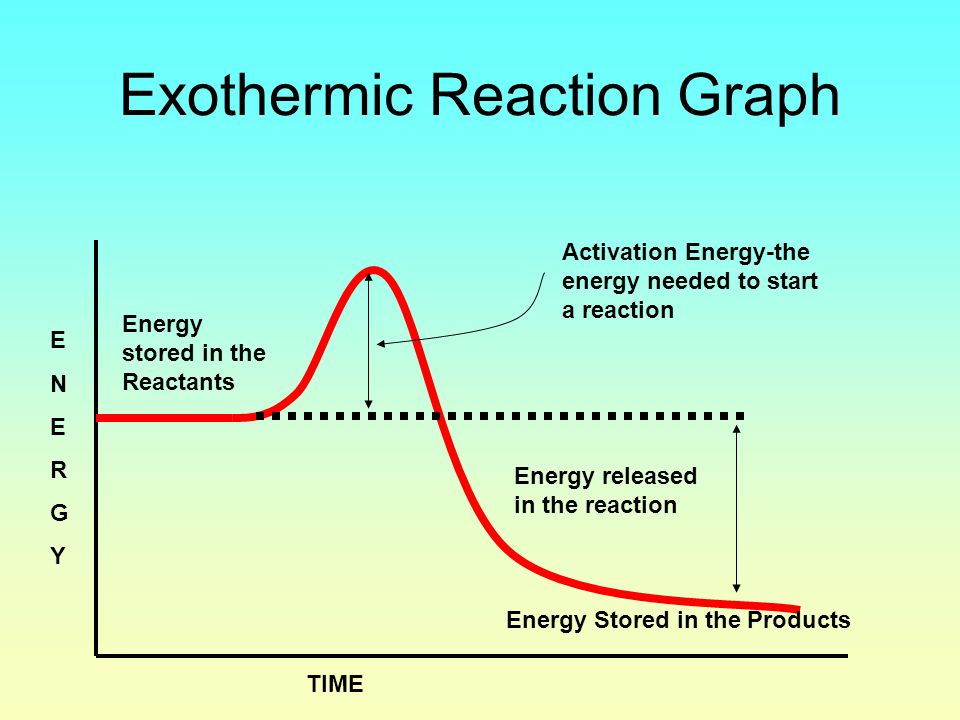
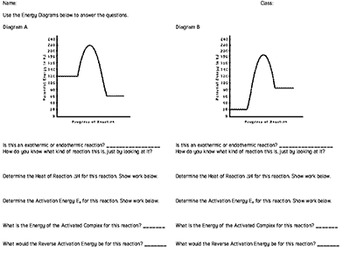
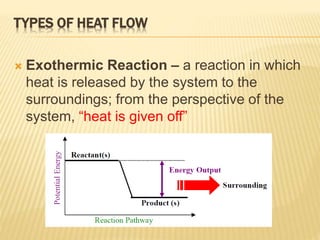
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... EXOTHERMIC Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative ... Endothermic vs Exothermic Reactions | ChemTalk You can think about this visually using a reaction energy diagram, as seen below: And endothermic reaction (left) and an exothermic reaction ...
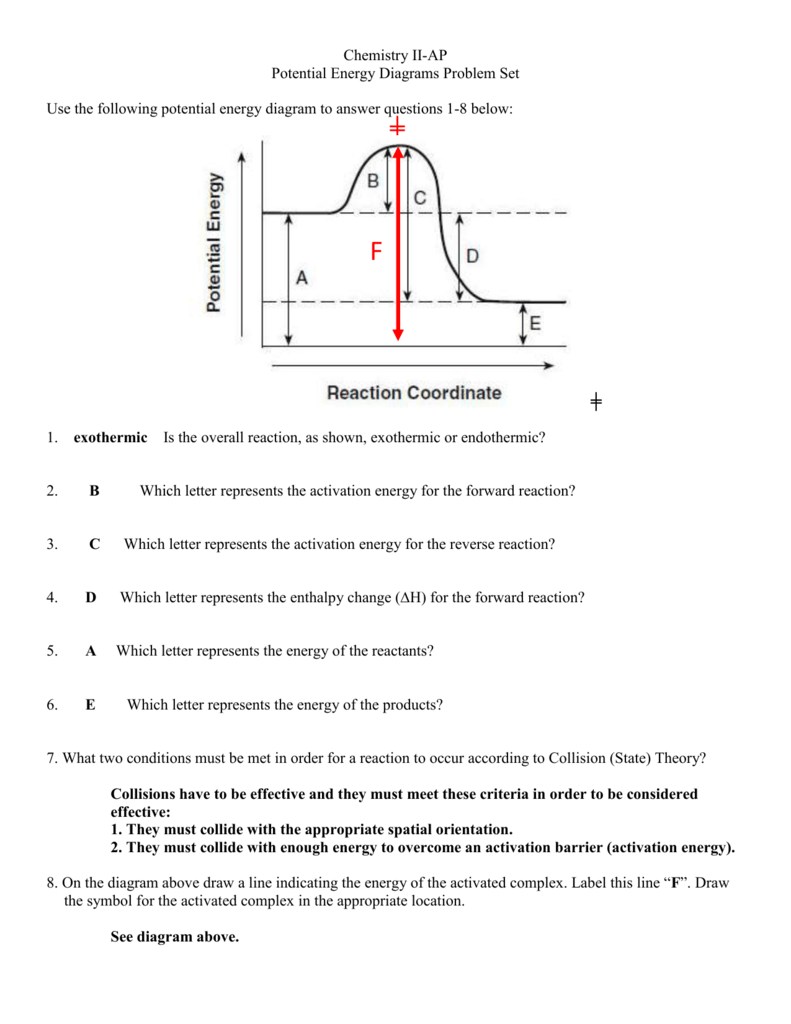
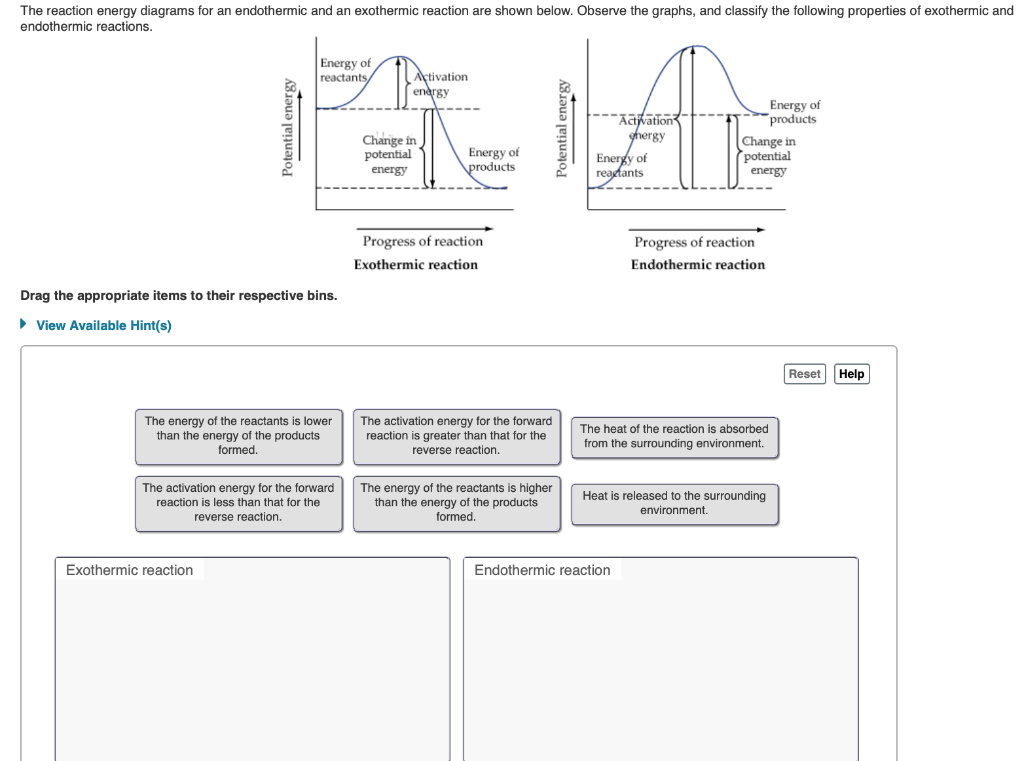
Solved The reaction energy diagrams for an endothermic and ... The heat of the reaction is absorbed from the surrounding environment. The activation energy for the forward reaction is less than that for the reverse reaction. The energy of the reactants is higher than the energy of the products formed. Heat is released to the surrounding environment. Exothermic reaction Endothermic reaction
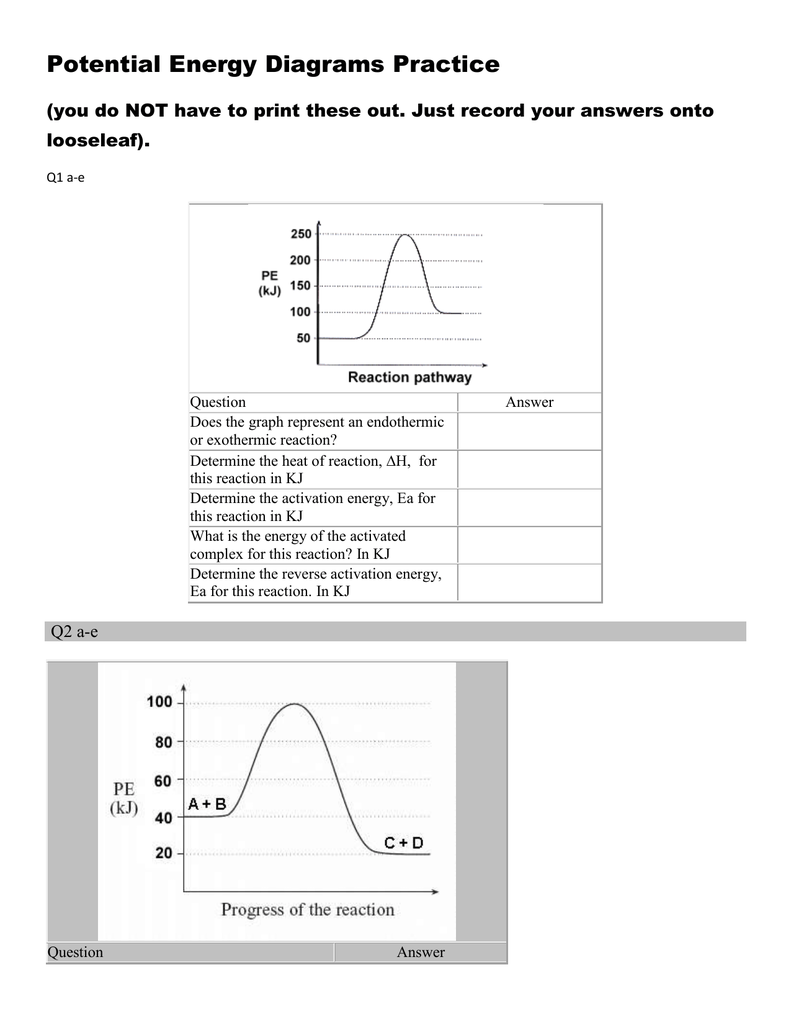
Energy diagram endothermic and exothermic reaction
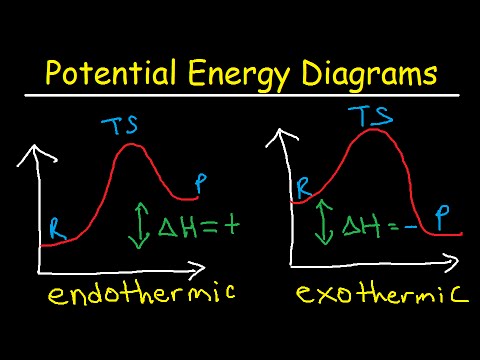
1. Draw the energy diagram for an endothermic | Chegg.com Chemistry. Chemistry questions and answers. 1. Draw the energy diagram for an endothermic reaction and exothermic reaction. Label everything including transition states and intermediates. Draw the energy diagram for the reaction with catalyst. Question: 1. How does the energy level diagram show this reaction is ... Energy profile diagrams for endothermic and exothermic reactions Every chemical substance has a certain amount of chemical energy. This energy is given the symbol H and is different for different substances. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured. Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
Energy diagram endothermic and exothermic reaction. Potential Energy Diagrams | CK-12 Foundation Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ... Potential Energy Diagrams Chemistry Catalyst Endothermic ... Energy diagrams. exothermic reactions. endothermic reactions. example. 6.3 kinetic energy, heat transfer, and thermal equilibrium. 6.4 heat capacity and coffee cup calorimetry. 6.5 phase changes and energy. 6.6 introduction to enthalpy of reaction. 6.7 bond enthalpy and bond dissociation energy. PPTX Energy and Chemical Reactions - Boyertown Area School ... Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic - more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making Endothermic and exothermic reactions worksheet with ... Some of the worksheets for this concept are Potential energy diagram work answers, Endothermic exothermic reactions, Chemistry 12 work 1 2, Name per work introduction to specific heat capacities, Chemical reactions and energy, , Name kinetics potential energy diagrams, Name date per.
Difference Between Endothermic and Exothermic Reactions ... Similar is the case with the endothermic and exothermic reactions in Chemistry. These release energy in the form of sound, light, cold or heat. In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. On the other hand, an exothermic reaction releases energy into the surrounding of the system. Energy Diagrams of Reactions | Fiveable Energy Diagrams Physical or chemical processes can be described through energy diagrams. As mentioned before, reactions can be categorized as endothermic or exothermic processes. The energy diagrams below show what should be known for the test. Image Courtesy of Pinterest Before looking at the specifics of each, you should be aware of a few terms: PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... c) Draw a labeled enthalpy level diagram for an exothermic and endothermic reaction showing the activation energy, Ea and enthalpy change. [4] 9. (M05/S/2) In a neutralization reaction 50 cm 3 of a 0.50 moldm-3 solution of sodium hydroxide is mixed rapidly in a glass beaker with 50 cm 3 of a 0.050 moldm-3 solution of sulfuric acid. 18.4: Potential Energy Diagrams - Chemistry LibreTexts Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
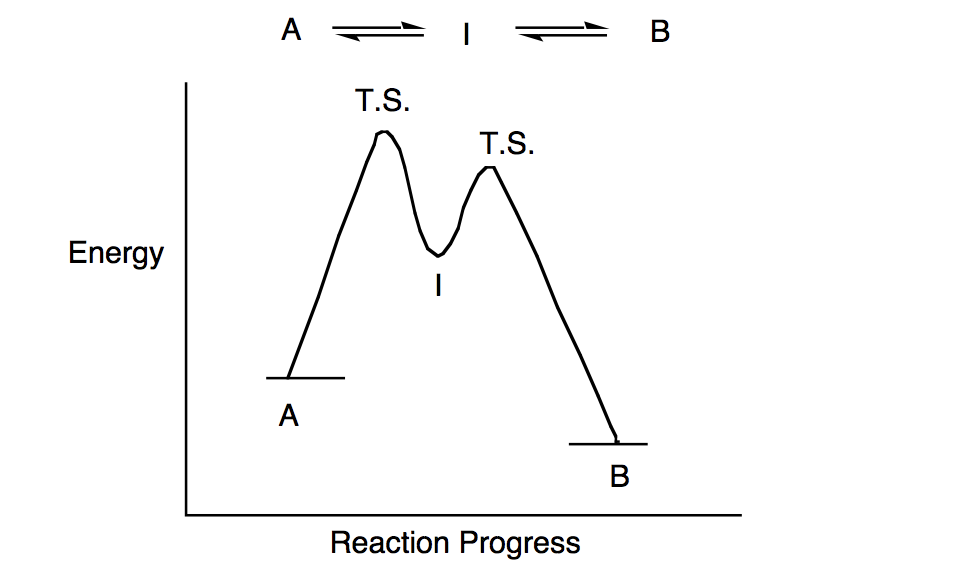
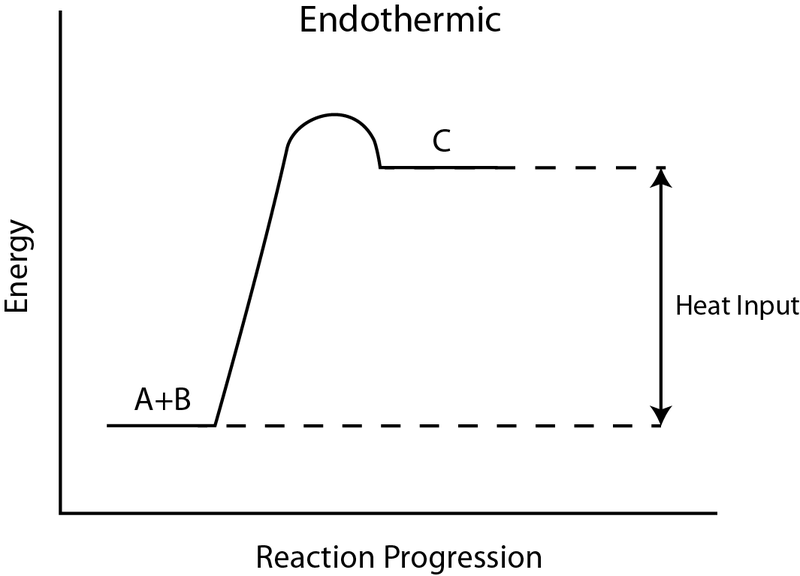
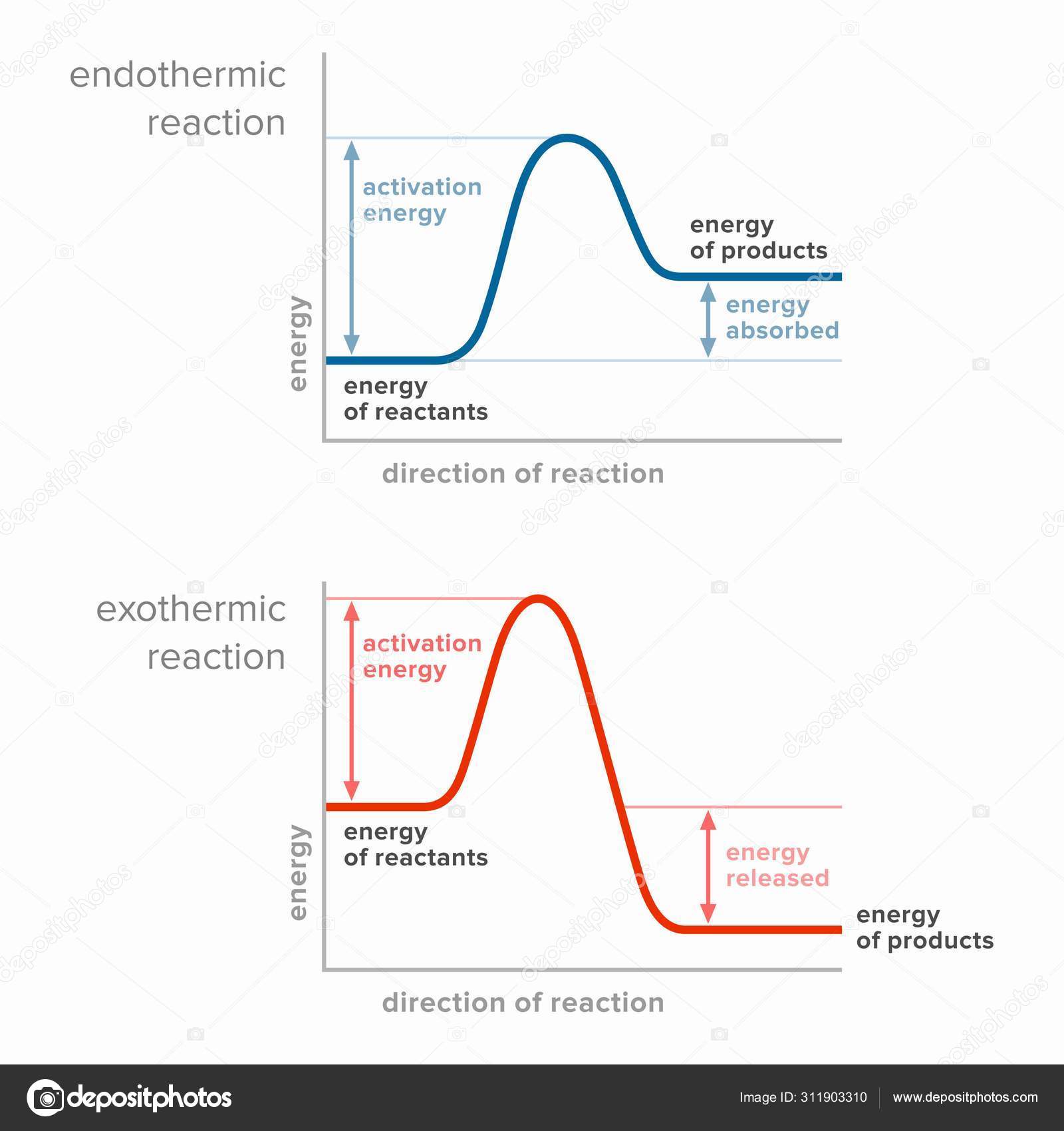
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. Creative Diagram Of Exothermic Reaction - Glaucoma Template Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure PageIndex2. As we can see and examine from the graph in an exothermic reaction the reactants are usually at a higher. In this type of reaction energy in the form of heat sound or light is released when the reactants break apart. PDF 5.1 - Exothermic and Endothermic Reactions 5.1 - Exothermic and Endothermic Reactions 5.1.1 - Define the terms exothermic reaction, endothermic reaction and standard enthalpy change of reaction Exothermic Reaction - A reaction that causes the temperature of the surroundings to increase. Energy is lost, or released, in the reaction, as the enthalpy of the products is less 9.5: Endothermic and Exothermic Reactions - Chemistry LibreTexts Energy Diagrams. Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\). In endothermic reactions, the energy of the reactants is lower than that of the products. This type of reaction is represented by an "uphill" energy-level diagram shown in Figure \(\PageIndex{2A}\).
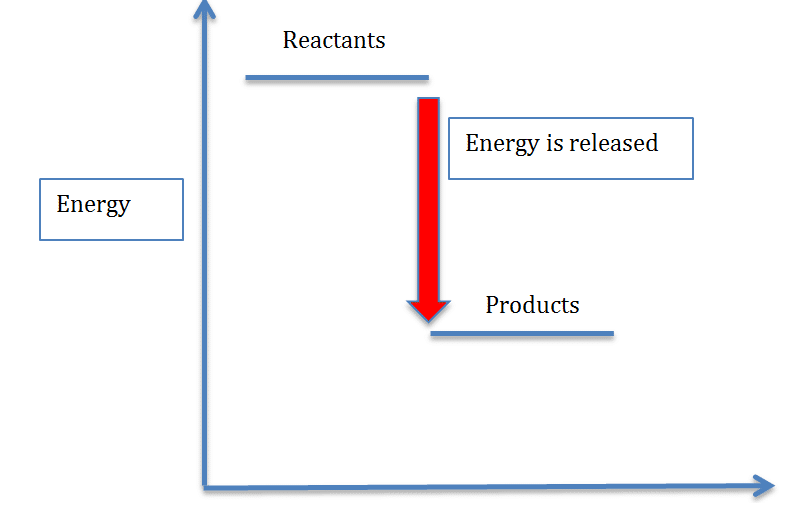
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. This means that there was more energy in reactants than in the products.
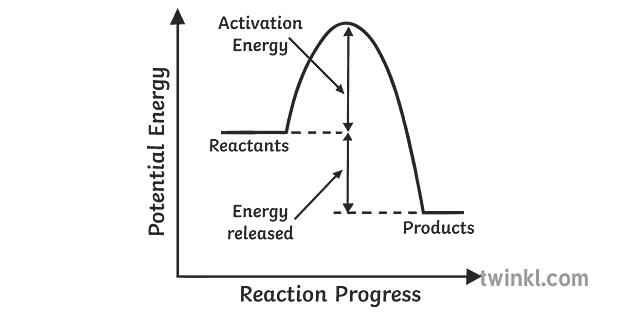
EXOTHERMIC & ENDOTHERMIC REACTIONS: ENERGY DIAGRAMS NOTICE THAT IN AN ENDOTHERMIC REACTION: • THE ACTIVATION ENERGY OF THE REVERSE REACTION IS LESS THAN. THAT OF THE FORWARD REACTION.
Exothermic, Endothermic, & Chemical Change | Energy ... Identifying Exothermic & Endothermic Reactions. There are two methods for distinguishing between exothermic and endothermic reactions. Monitor temperature change. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the temperature decreases.
Exothermic and endothermic reactions - Energy changes in ... Exothermic and endothermic reactions When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change. For example, when a bonfire burns it...
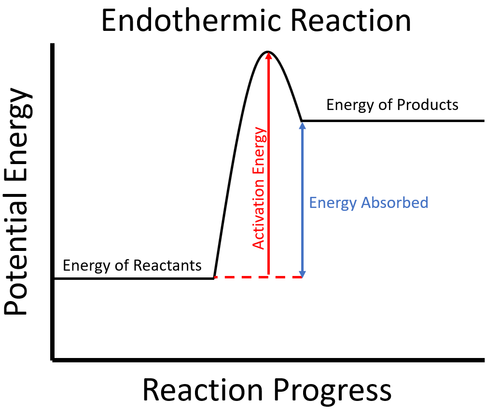
Endothermic Reactions: Definition, Example, Diagram and ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
Draw the energy diagram for an endothermic and exothermic ... Reaction energy diagrams show how the energy of a system changes as the reactants form the products. On the diagram, we can label the activation energy and the ...
Representing endothermic and exothermic processes using energy ... A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the ...
Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
Energy in Chemical Reactions I Unit Test Flashcards | Quizlet It is an endothermic reaction because the system takes energy from the surroundings. A student pours ammonium nitrate into a container of room temperature water. The student stirs the mixture with a stirring rod and then feels the outside of the container, which is cold to the touch.
Chemistry Study Sheet Mod 4.pdf - Energy Changes in ... Energy Changes in Chemical reactions: 14.1 Exothermic and endothermic reactions: - Chemical energy is stored in the bonds between atoms and molecules + Energy is measured in Joules (J), kJ or MJ + 1 J = 0.001 kJ = 0.000001 MJ + Energy is conserved in chemical reactions - cannot be created or destroyed - Chemical reactions and changes of state involve energy changes - energy leaves the system ...
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Reaction Coordinate Diagram Endothermic Vs Exothermic Oct 01, 2019 · Endothermic vs. exothermic reactions. “When trying to classify a reaction as exothermic or endothermic, watch how the temperature of the surrounding—in this case, the flask—changes. the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less.
Difference between Exothermic and Endothermic Reactions ... The major difference between endothermic and exothermic reactions, as their names suggest, is that the former absorbs heat from the surroundings while the latter releases it. Endothermic Reactions - The term "endothermic reaction" refers to a process in which a system absorbs energy in the form of heat from its surroundings.
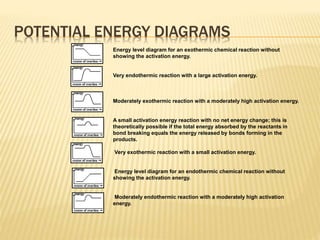
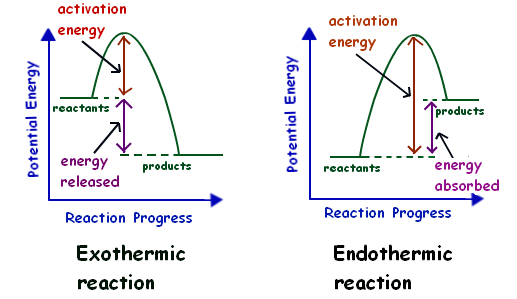
Potential Energy Diagrams - Kentchemistry.com Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products.
Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
PDF Exothermic And Endothermic Reactions In Everyday Life Energy diagrams for endothermic and exothermic reactions. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants. Endothermic vs. exothermic reactions (article) | Khan Academy
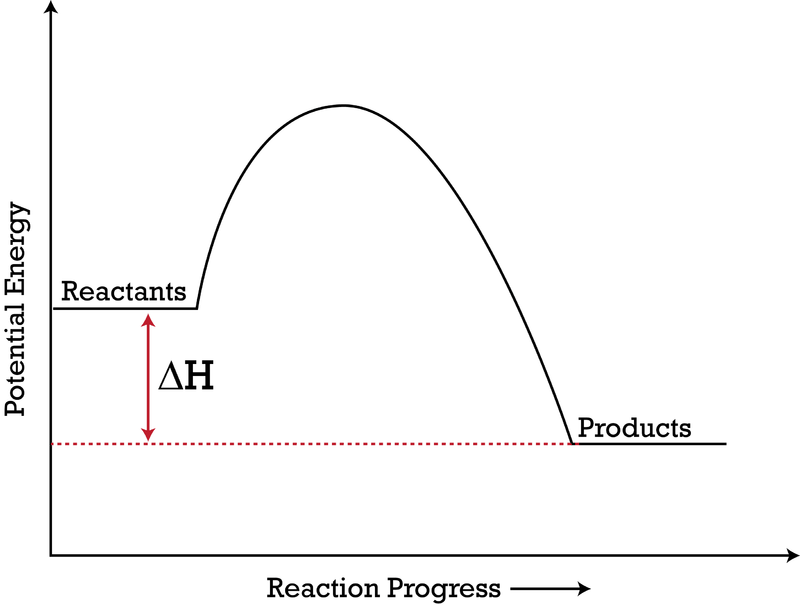
How do you know if a diagram is endothermic or exothermic ... How do you know if a diagram is endothermic or exothermic? In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0).
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
How does the energy level diagram show this reaction is ... Energy profile diagrams for endothermic and exothermic reactions Every chemical substance has a certain amount of chemical energy. This energy is given the symbol H and is different for different substances. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be easily measured.
1. Draw the energy diagram for an endothermic | Chegg.com Chemistry. Chemistry questions and answers. 1. Draw the energy diagram for an endothermic reaction and exothermic reaction. Label everything including transition states and intermediates. Draw the energy diagram for the reaction with catalyst. Question: 1.

















0 Response to "38 energy diagram endothermic and exothermic reaction"
Post a Comment