35 nf molecular orbital diagram
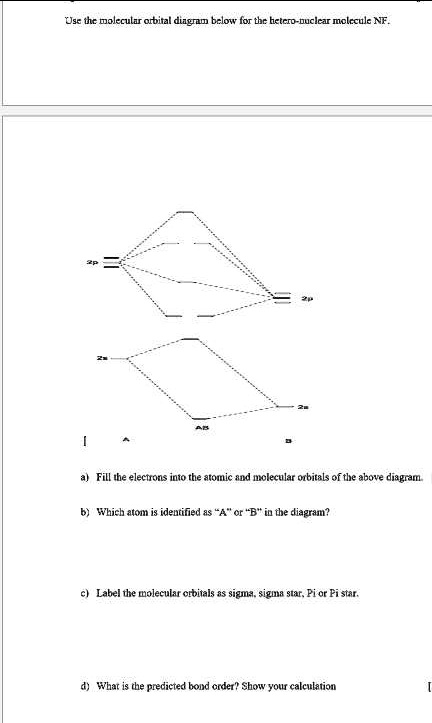
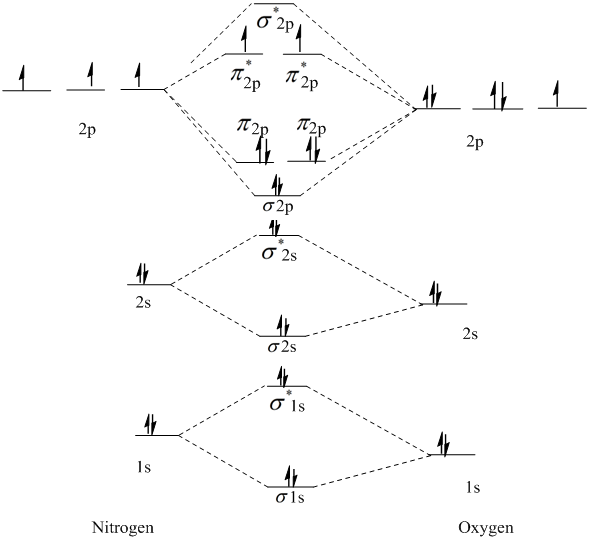
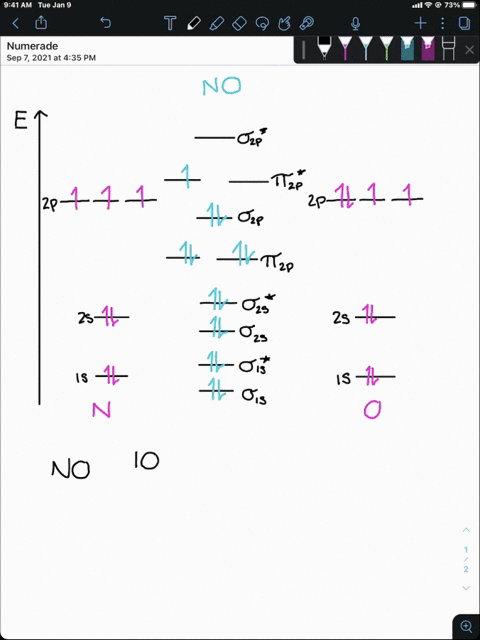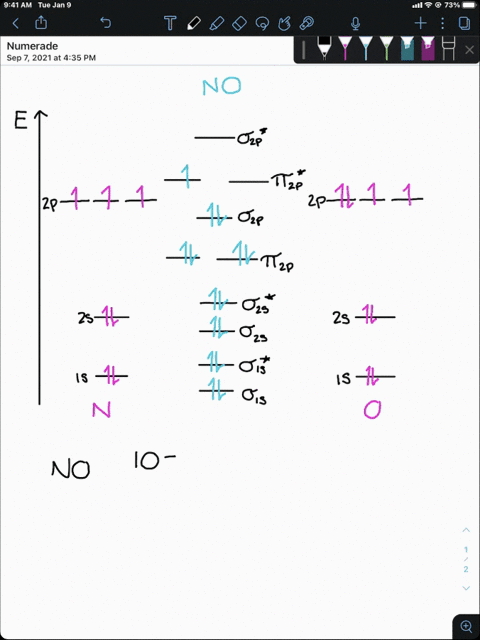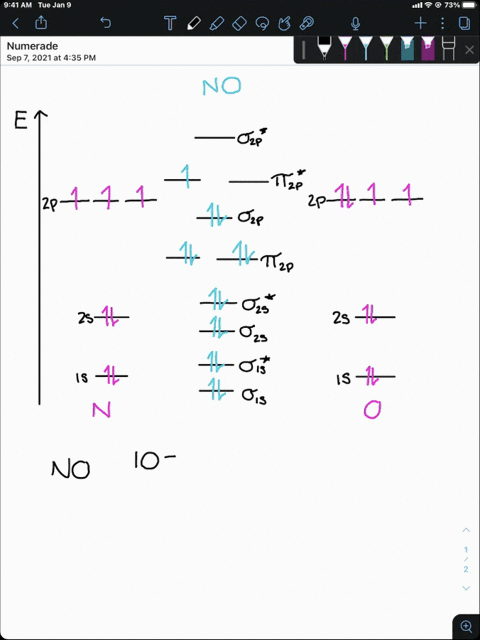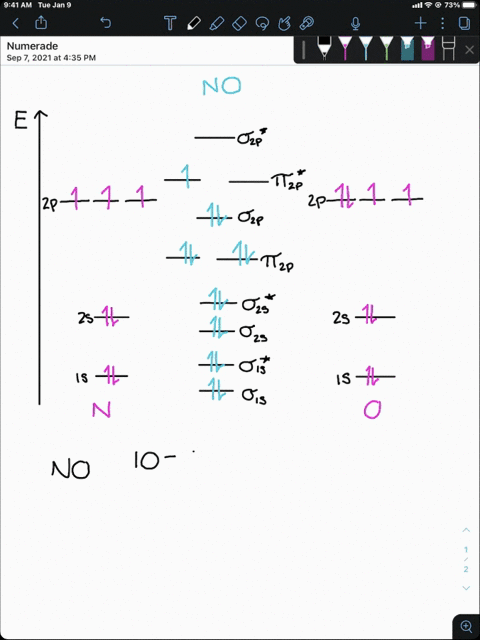
Solved a) Complete the molecular orbital diagram for NF ... a) Complete the molecular orbital diagram for NF, nitrogen monofluoride below. Electrons can be added to the orbitals by clicking the orbital. Additional clicks will change the occupation of the orbital. Be sure to fill in both the atomic and molecular orbitals. Note: atomic orbitals are not shown. 629 2p 2p Tap 028 02 2s 2s 025 N F b) Using ... Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
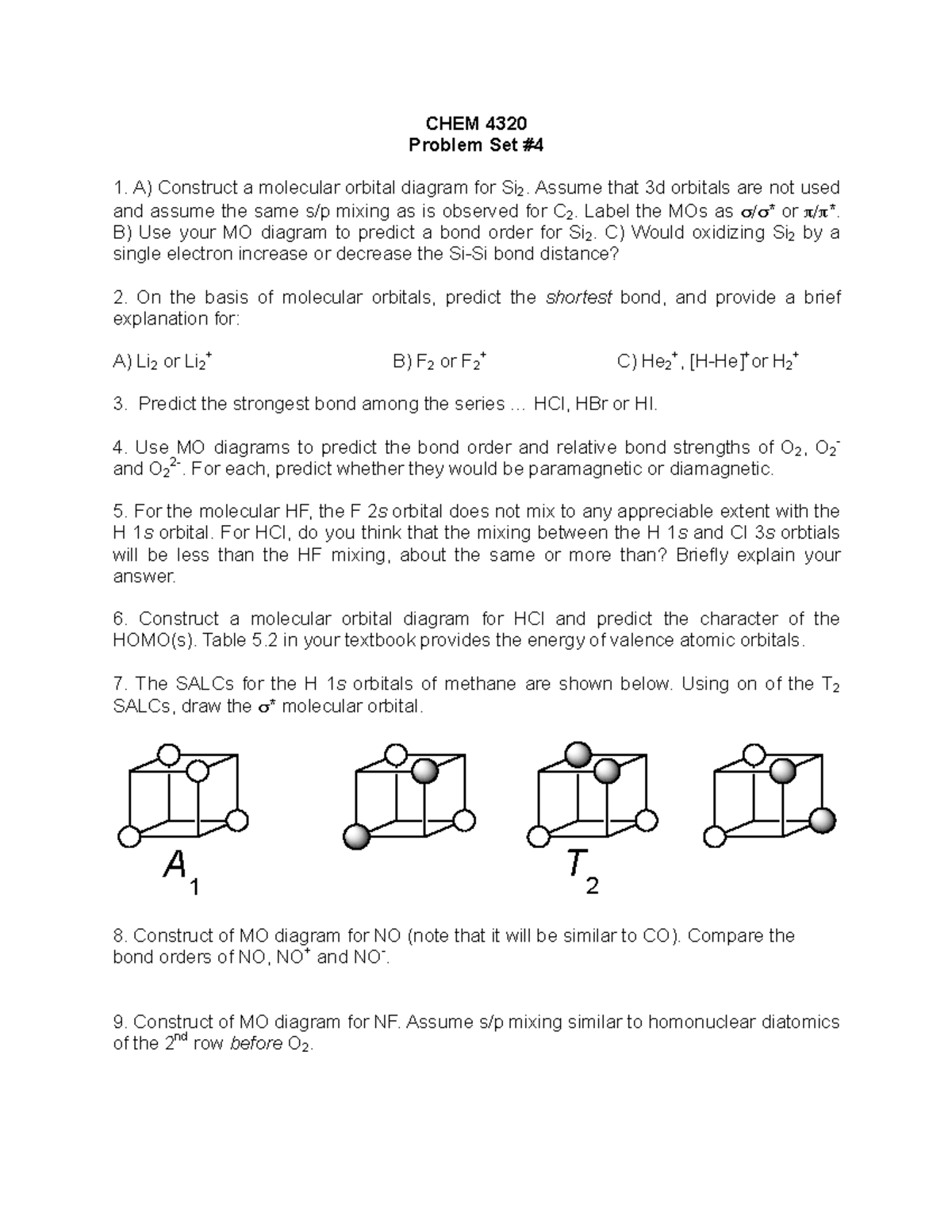
Nf molecular orbital diagram
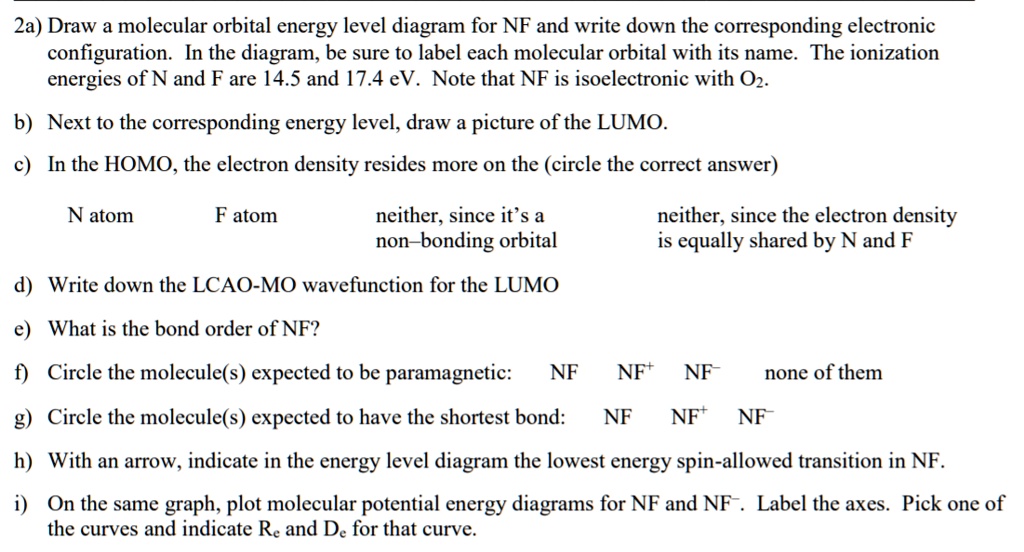
NF is a known molecule! Construct a molecular orbital diagram ... NF is a known molecule! Construct a molecular orbital diagram for NF, an include sketches showing how the valence orbitals of N and F interact to form MOs. What is the likely bond order for NF?... CHEM 1411. Chapter 8.Molecular Geometry and Bonding ... Chapter 8.Molecular Geometry and Bonding Theories (Homework) W molecule. ____ 13. Four of the following statements about the ammonia molecule, NH 3, and the nitrogen trifluoride molecule, NF 3, are correct. One is not. Which one? a. The nitrogen atom can be described as utilizing sp3 hybrid orbitals in the nitrogen trifluoride molecule. b. Configuration interaction studies of NF and NF - ScienceDirect In Table 5 we give the dominant electron configuration for each state in terms of natural orbitals (15), i.e., the orbitals which diagonalize the first order reduced density matrix. These orbitals are in this case molecular orbitals, which can be given in terms of the raw atomic Slater type basis, as is done in Table 6 for the X 3-'- state of NF.
Nf molecular orbital diagram. NF3 Lewis Structure, Molecular Geometry, Hybridization ... As we can see, the above diagram gives us the Periodic table. Nitrogen belongs to group 15 and fluorine, being a halogen, belongs to group 17. nitrogen has 5 and fluorine has seven valence electrons. The total number of valence electrons in a NF3 molecule = 5 + 7*3 = 26. 8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. PDF Hybridization and Molecular Orbital (MO) Theory overlap of atomic orbitals to form molecular orbitals, electrons are then distributed into MOs. A molecule is a collection of ... • NH 3 and NF 3 are trigonal pyramidal, polar molecules. Steps in predicting the hybrid orbitals used by an atom in bonding: 1. Draw the Lewis structure 2. Determine the electron pair geometry using the VSEPR model Main axis of diatomic molecule is z. The orbitals px and ... Which of the following combination of orbitals will form a nonbonding molecular orbital ? ©® X-X 37. Correct order for N O hond length in the given. Solve Study Textbooks ... What is the preffered orientation of one NF molecular realtive to other ? Medium. View solution > View more. More From Chapter. Chemical Bonding and Molecular Structure ...
Chemical Bonding and Molecular Structure Class 11 ... In an anti-bonding molecular orbital, electron density is minimum. (a) around one atom of the molecule. (b) between the two nuclei of the molecule. (c) at the region away from the nuclei of the molecule. (d) at no place. Answer. B. Question. When two atomic orbitals combine, they form. Molecular Orbital (MO) Diagram of N2 - YouTube Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or... NF MO Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... PDF Answers to Molecular Orbitals Problem Set ANSWERS TO MOLECULAR ORBITALS PROBLEM SET 1. (a) N2 +(13 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22pσ12p N2 2+(12 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22p N2 (14 e-): σ2 1sσ*21sσ22sσ*22sπ22pπ22pσ22p N2-(15 e-): σ21sσ*21sσ22sσ*22sπ22pπ22pσ22pπ*12p N2 2-(16 e-): σ21sσ*21sσ22sσ*22sπ22pπ22pσ22pπ*12pπ*12p (b) Bond orders are: N2 + = 2.5 ; N 2 2+ = 2.0 ; N
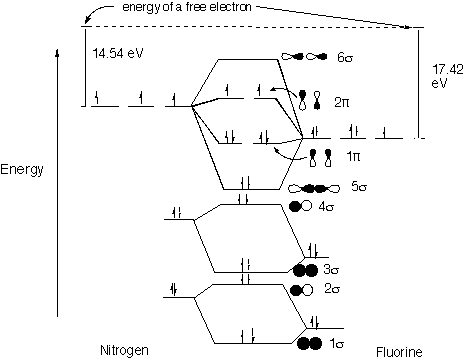
Solved: Chapter 5 Problem 9P Solution | Inorganic ... Step-by-step solution. 89% (9 ratings) for this solution. Step 1 of 4. a) The electron configurations of nitrogen and fluorine are as follows: The valence orbitals (such as and orbitals) of N and F interact to form a molecular orbital of. The energy levels of oxygen are low lying since oxygen is more electronegative than nitrogen. Configuration interaction studies of NF and NF+ ... Full potential energy curves and wavefunctions are calculated for NF and NF + in the Born-Oppenheimer approximation with a configuration interaction method employing a minimal basis of Slater type orbitals. Resulting energy differences, vibrational and rotational constants are compared to experimental results and predictions are made of these quantities for states which have not yet been ... Miessler-Fischer-Tarr5e SM Ch 05 CM Therefore, NF is predicted to be paramagnetic with a bond order of 2. The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * Exam224.pdf - Question: NF is a known molecule! a ... Question: NF is a known molecule! a. Construct a molecular orbital energy-level diagram for NF, being sure to include sketches that show how the valence orbitals of N and F interact to form molecular orbitals. b. What is the most likely bond order for NF? c. What are the point groups of the molecular orbitals of this molecule?
Molecular Orbital Diagram for NF - CHEMISTRY COMMUNITY Nov 05, 2015 · I have a quick question about the molecular orbital diagram for NF. Lavelle said that whenever we have a heteronuclear molecule, if one or both atom (s) has Z<8, then we would use the diagram where the pi px and pi py are lower than the Sigma pz. However, I've also heard of a method where you can average the Z (so in NF the Z would be 7+9=16/2=8).
SOLVED:Bonding and Molecular Structure: Orbital ... Draw the Lewis structure for $\mathrm{NF}_{3} .$ What are its electronpair and molecular geometries? What is the hybridization of the nitrogen atom? What orbitals on $\mathrm{N}$ and $\mathrm{F}$ overlap to form bonds between these elements? ... Assume the molecular orbital diagram for a homonuclear diatomic molecule (Figure 10.22 ) applies to ...
Chem 32 Virtual Manual - Stanford University 4.* (1997 1 7) Consider the diatomic molecule NF. Draw its molecular orbital energy diagram. A. Using the LCAO-MO scheme, indicate the ground-state MO description for NF, i.e. complete the following: 1(s) 2. . . The LCAO-MO description is: 1s 2 2s 2 3s 2 4s 2 5s 2 1p 4 2p 2. B. What is the multiplicity of the ground state of NF?
Molecular Orbital Diagram Maker - University of Sydney ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
PDF 09-107 Honors Chemistry Carnegie Mellon University Exam 2 a) [6 pts] Draw the MO diagram for the NF molecule (order the molecular orbitals as you would in F2). Include the relative energies of the atomic orbitals, fill in the electrons and label the molecular orbitals. b) [4pts] What is the bond order for this molecule? Bond order = _____ c) [3pts] Is the molecule paramagnetic or diamagnetic?
4 Draw the valence molecular orbital diagram for NF State ... 4. Draw the valence molecular orbital diagram for NF. State the bond order, the molecular orbital configuration and determine whether each of the following molecules/ions is paramagnetic or diamagnetic. (6 points) a) Molecular Orbital Diagram b) Bond Order _____ c) Molecular orbital configuration _____ d) Consider NF, NF +, and NF-. Which of ...
pi 2p bonds or sigma 2p bonds - CHEMISTRY COMMUNITY In discussion we drew the molecular orbital diagram for OF and NF. In the OF diagram, the sigma 2p bond is below the pi 2p bond (and vice versa for the NF diagram). ... the sigma 2p orbital is lower in energy than the pi 2p orbitals. Z is the atomic number or equivalently the number of protons in the nucleus. Top. Alex Yee - 4I Posts: 15
Answered: Draw a molecular orbital diagram for NF… | bartleby Science Chemistry Q&A Library Draw a molecular orbital diagram for NF and calculate the bond order. Also, comment on its magnetic property. Also, comment on its magnetic property. Draw a molecular orbital diagram for NF and calculate the bond order.
PDF Chapter 9 Chemical Bonding II: Molecular Geometry and ... 9.6 Molecular Orbital Theory •Molecular Orbital Theory (MO) -Atomic orbitals combine to form new molecular orbitals which are spread out over the entire molecule. Electrons are in orbitals that belong to the molecule as a whole. -Molecular orbitals (wave functions) result from adding and/or subtracting atomic orbitals (wave functions).
Configuration interaction studies of NF and NF - ScienceDirect In Table 5 we give the dominant electron configuration for each state in terms of natural orbitals (15), i.e., the orbitals which diagonalize the first order reduced density matrix. These orbitals are in this case molecular orbitals, which can be given in terms of the raw atomic Slater type basis, as is done in Table 6 for the X 3-'- state of NF.
CHEM 1411. Chapter 8.Molecular Geometry and Bonding ... Chapter 8.Molecular Geometry and Bonding Theories (Homework) W molecule. ____ 13. Four of the following statements about the ammonia molecule, NH 3, and the nitrogen trifluoride molecule, NF 3, are correct. One is not. Which one? a. The nitrogen atom can be described as utilizing sp3 hybrid orbitals in the nitrogen trifluoride molecule. b.
NF is a known molecule! Construct a molecular orbital diagram ... NF is a known molecule! Construct a molecular orbital diagram for NF, an include sketches showing how the valence orbitals of N and F interact to form MOs. What is the likely bond order for NF?...

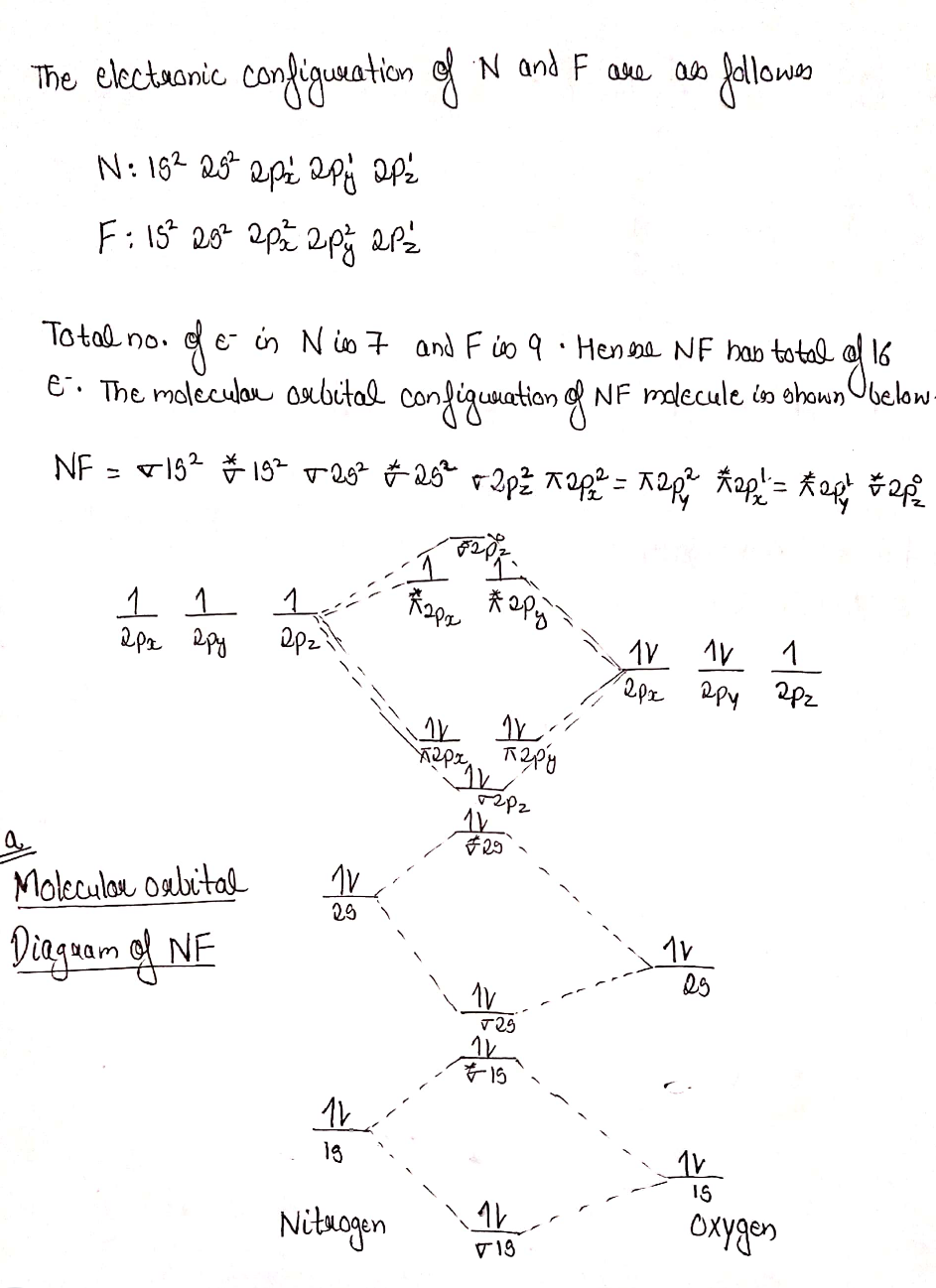














0 Response to "35 nf molecular orbital diagram"
Post a Comment