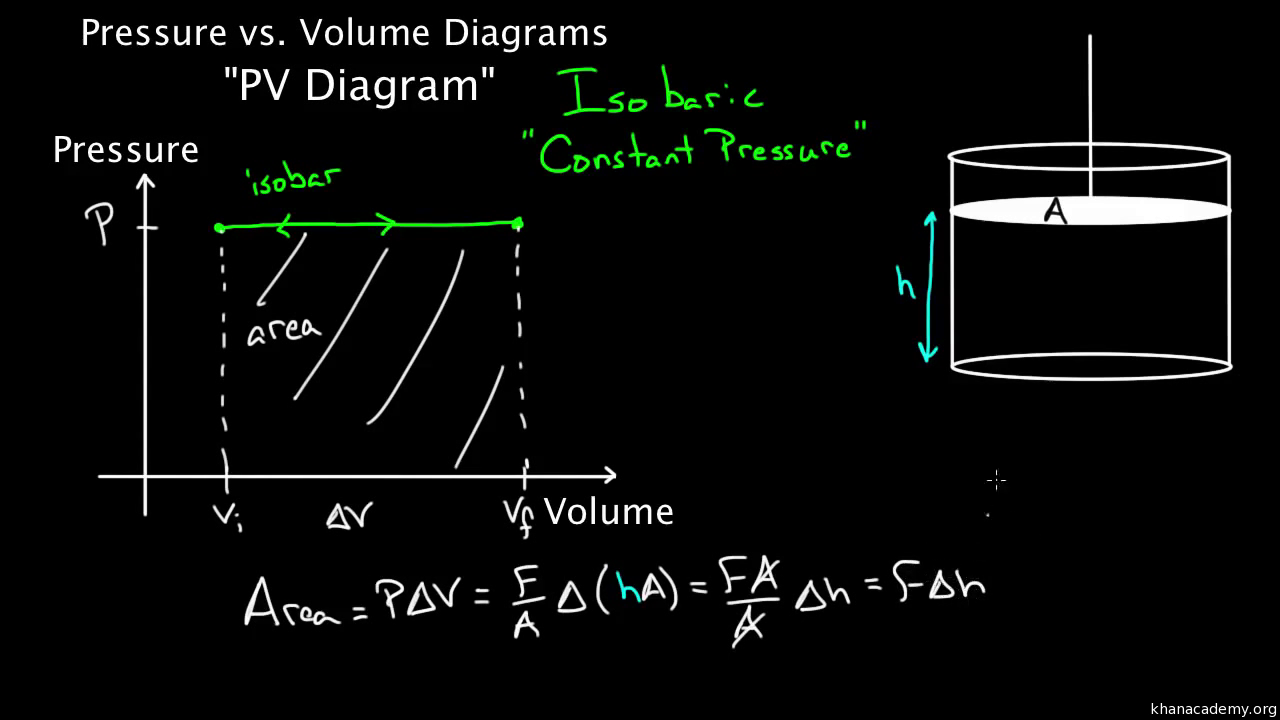
38 one way to increase the volume of the gas in the balloon in the diagram above is to -
Volume and pressure in gases - the gas laws Boyle's law. Decreasing the volume of a gas increases the pressure of the gas. An example of this is when a gas is trapped in a cylinder by a piston. A quantity of gas is contained in a sealed container of fixed volume. The temperature of the gas is increased. (b) ... The volume of the balloon is 4800m3. The pressure of the helium is 98kPa. (c) ... draw a diagram. ...
What is the density of a substance with a mass of 15 grams and a volume of ... The graph above illustrates how the viscosity of a liquid changes with ...

One way to increase the volume of the gas in the balloon in the diagram above is to -
May 25, 2015 · 2 answersIf the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated. Let's look at two simpler cases ... One way to increase pressure on a gas is to a. decrease temperature b. increase volume c. increase the number of gas particles d. lower the kinetic energy of the gas molecules 4. How do gas particles respond to an increase in volume? a. ... the highest point above the ground, the pressure on the balloon is 35.0 kPa. What is the new volume of A balloon is filled with helium gas. For the question(s) that follow, select the letter of the balloon diagram that corresponds to the given change in conditions. The balloon is put into a chamber whose pressure is less than the atmospheric pressure and at atmospheric temperature. A B C A and B B and C
One way to increase the volume of the gas in the balloon in the diagram above is to -. The ideal gas law equation tells us that the pressure of the air in the balloon will increase. The increase is momentary though. Because the pressure inside is now greater (the big yellow arrows) than the pressure outside, the balloon will expand. As volume begins to increase, the pressure of the air inside the balloon will decrease. The diagram above is a molecular model of a gaseous diatomic element that is just above its boiling point. Intermolecular forces between the gas molecules will cause them to condense into the liquid phase if the temperature is lowered. The diagram above shows a plot of the results. ... 1. The molar volume of an ideal gas in liters at STP is - ... The gas in balloon B is warmer. Rating: 4 · 1 review One common science homework question is to list three ways to increase the pressure of a gas container or a balloon. This is an excellent question because answering it helps you understand what pressure is and how gases behave.
A student wants to study the effects of volume on gas pressure. During his experiment, he recorded the above data. How could he now study the effects of.19 pages Represented above are five identical balloons, each filled to the same volume at 25 C and 1.0 atmosphere pressure with the pure gases indicated. (a) Which balloon contains the greatest mass of gas? Explain. (b) Compare the average kinetic energies of the gas molecules in the balloons. Explain. Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste · 2016 · ScienceAs you increase the temperature of a gas in a sealed, rigid container, ... Thus the volume of the balloon increases, making the density smaller. One way to increase the volume of the. gas in the balloon in the diagram. above is to — F . cool the gas in the balloon only. G . increase the temperature of the water _ H . push the balloon farther down into the. water bath. J . seal the top of the water bath. One of the main assumptions of the. kinetic molecular theory of gases is. that the ...
The diagram below shows gas inside a sealed container before and after the force is applied to the container's movable piston. The temperature inside the container remains the same after the force is applied. Applying force to the piston results in compression of the gas particles and an increase in gas pressure. The balloon is going to look somewhat deflated to due to the decrease in volume. Temperature and gas volume are directly proportional, so if one decreases so does the other. But there will always be some volume so it could not have been completely deflated. Which is a correct way of stating Boyle's law? P=k×1V, where k is a constant. PV=k, where k is a constant. P1V1=P2V2, where the indices 1 and 2 corresponds to different states of the same gas sample. all of the above If we put the balloon in a refrigerator, the gas inside gets cold and the ... shows how cooling and heating a gas causes its volume to decrease or increase, ...
A balloon is filled with helium gas. For this question, select the letter of the balloon diagram that corresponds to the given change in conditions. The balloon is put into a chamber whose pressure is less than the atmospheric pressure and at atmospheric temperature. a. 335 torr b. 432 torr c. 760. torr d. 1.44 torr e. 1 110 torr
5. _____ One way to increase the volume of the gas in the balloon in the diagram below is to - a. cool the gas and the balloon only b. increase the temperature of the water c. push the balloon farther down into the water bath d. seal the top of the water bath 6. _____ One of the main assumptions of the kinetic molecular theory
Consider a gas in a cylinder at room temperature (T = 293 K), with a volume of 0.065 m 3. The gas is confined by a piston with a weight of 100 N and an area of 0.65 m 2. The pressure above the piston is atmospheric pressure. (a) What is the pressure of the gas? This can be determined from a free-body diagram of the piston.
To increase the volume of a gas * reduce the pressure, or * increase the temperature, or * add more gas When 0.549 moles of a certain gas fill a balloon the volume is 75 mL.
(e) The lifting capacity of a hot air balloon is equal to the difference in the mass of the cool air displaced by the balloon and the mass of the gas in the balloon. What is the difference in the mass of 1.00 L of the cool air in part (c) and the hot air in part (d)? (f) An average balloon has a diameter of 60 feet and a volume of 1.1 × 10 5 ...
When one pushes up on the diaphragm, the balloons deflate completely, and when one pulls down on the diaphragm, they inflate. The physical principle behind this is known as Boyle's law, which states that at constant temperature, for a fixed amount of gas, pressure and volume are inversely proportional, or PV= k .
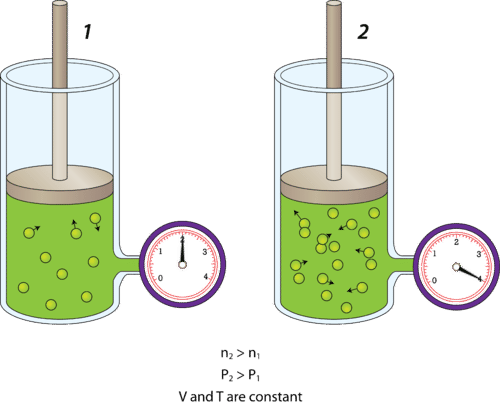
Volume of the gas increases; A common example of this can be seen when you hold parties at home! When you try to inflate a balloon, you blow air into the balloon (as indicated by the arrow in the diagram below). This causes more air to enter the balloon, increasing the amount of matter in the balloon. Thus, the mass of the gas in the balloon ...
The kinetic energy is 12mv2+12 (5m) (3v)2=23mv212mv2+12 (5m) (3v)2=23mv2. A scientist has two well-insulated containers, one filled with atoms of ideal gas X and the other with atoms of ideal gas Y. The gas X atoms have mass m, and the gas Y atoms have mass 5m. The containers are then connected so that the gases mix together.
A balloon is filled with helium gas. For the question(s) that follow, select the letter of the balloon diagram that corresponds to the given change in conditions. The balloon is put into a chamber whose pressure is less than the atmospheric pressure and at atmospheric temperature. A B C A and B B and C
One way to increase pressure on a gas is to a. decrease temperature b. increase volume c. increase the number of gas particles d. lower the kinetic energy of the gas molecules 4. How do gas particles respond to an increase in volume? a. ... the highest point above the ground, the pressure on the balloon is 35.0 kPa. What is the new volume of
May 25, 2015 · 2 answersIf the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated. Let's look at two simpler cases ...





















0 Response to "38 one way to increase the volume of the gas in the balloon in the diagram above is to -"
Post a Comment