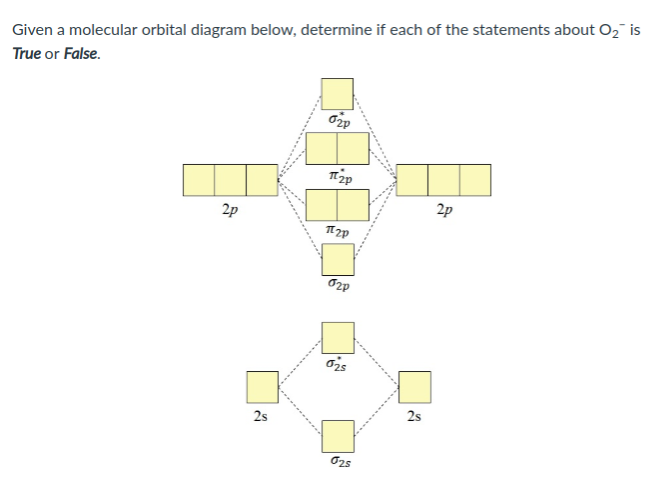
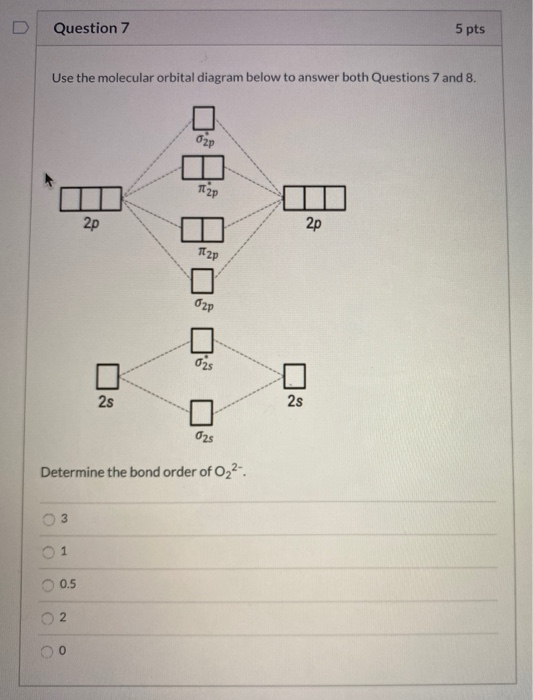
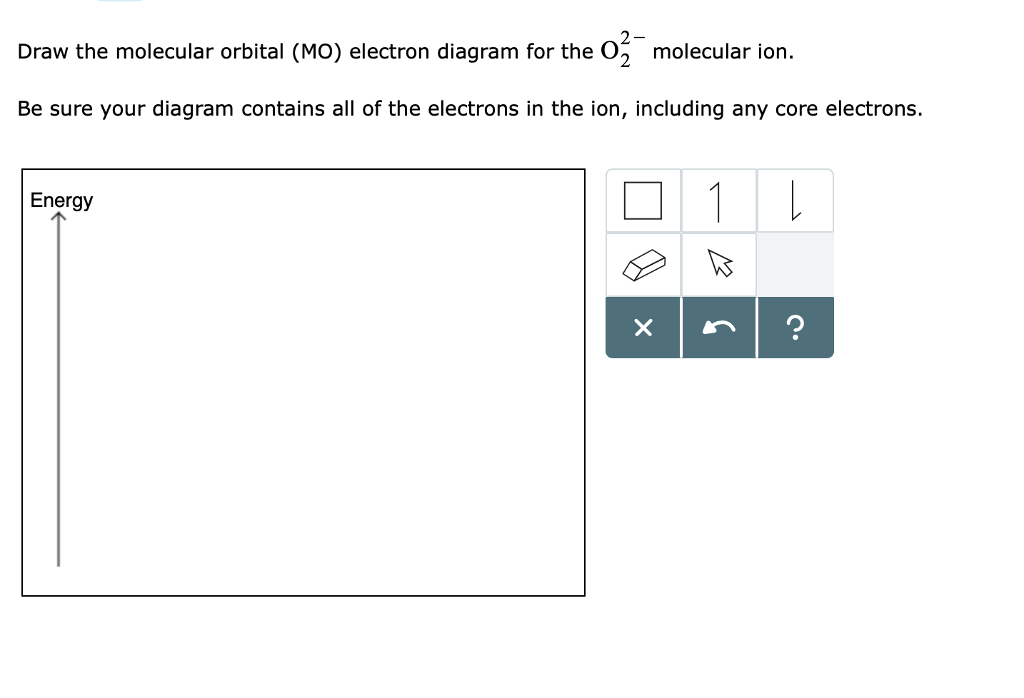
34 o2 2- molecular orbital diagram
Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. orbitals of most heavy metals allow them to form com- plex compounds in the cells, leading to toxic effects [ 52 ]. Although the intracellular concentration of metal ions
Jan 28, 2022 · Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital.
O2 2- molecular orbital diagram
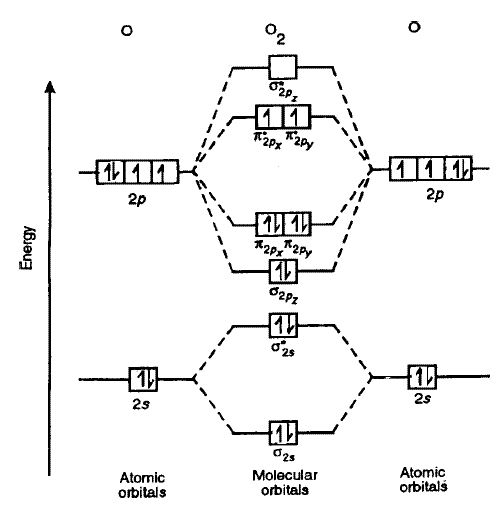
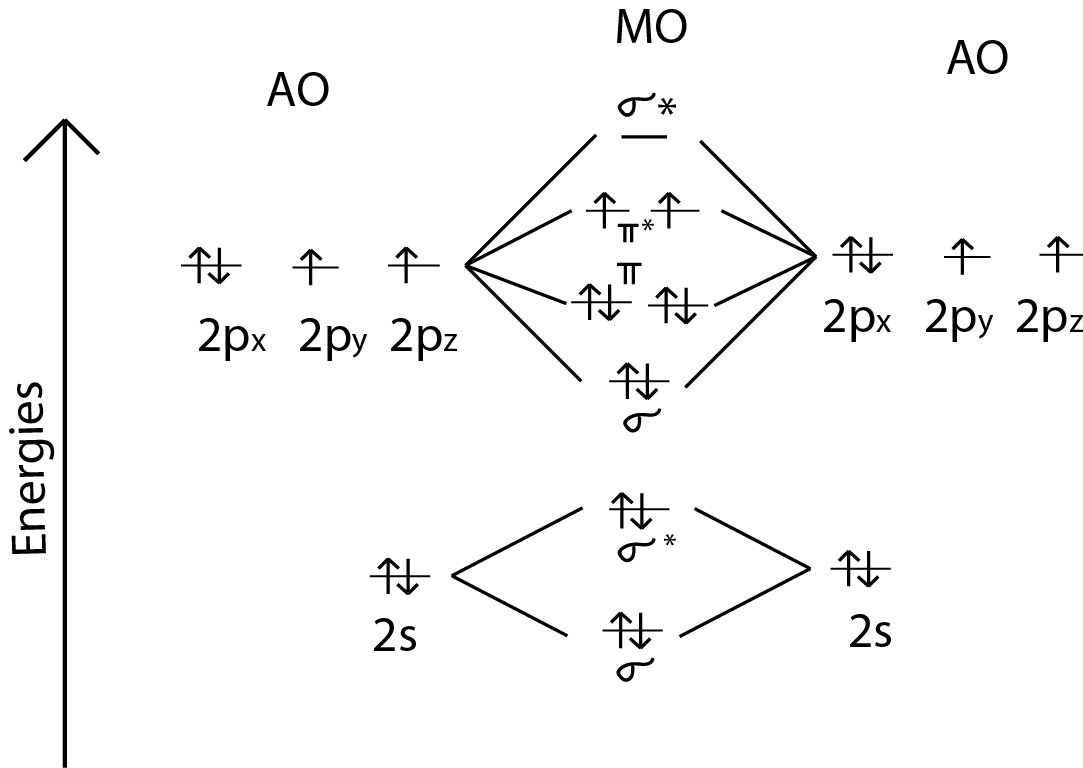
Jan 28, 2022 · The molecular orbital diagram shows the energy state at each level where the excited state increases from the bottom to the top. The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule. Mar 18, 2018 · "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z …
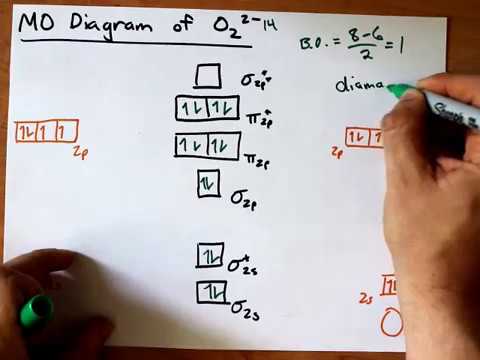
O2 2- molecular orbital diagram. Jan 04, 2022 · Ques.6: Use the molecular orbital energy level diagram to show that N 2 would be expected to have a triple bond, F 2 a single bond, and Ne 2 no bond. (5 Marks) Answer: N 2 Molecule = Electronic Configuration of N-atom (Z=7) is 1s 2 2s 2 2p x 1 2p y 1 2p z 1 . Feb 03, 2019 · sorry about that not being a molecular orbital diagram i saw orbital and immediately thought electron configuration to save confusion, could. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen. The other is for AFTER nitrogen. In chemistry, Molecular orbital (MO) theory is a method for describing the electronic structure of molecules using quantum mechanics. Electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.Main postulates of this theory are Atomic orbitals of comparable Formula. Bond order (B.O) 1/2 × [Number of an electron in antibonding molecular orbitals] – [Number of electrons in bonding molecular orbitals] The higher the order of the bond the greater the pull between the two atoms and the shorter the length of the bond. B.O for O 2– = 1.5.
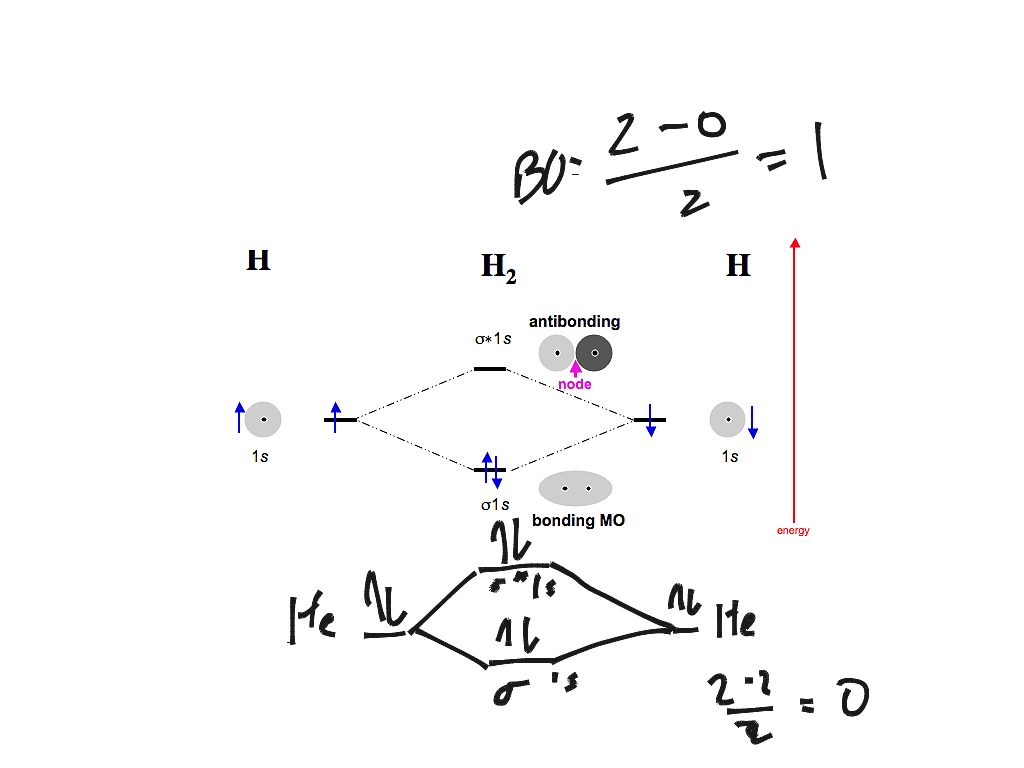
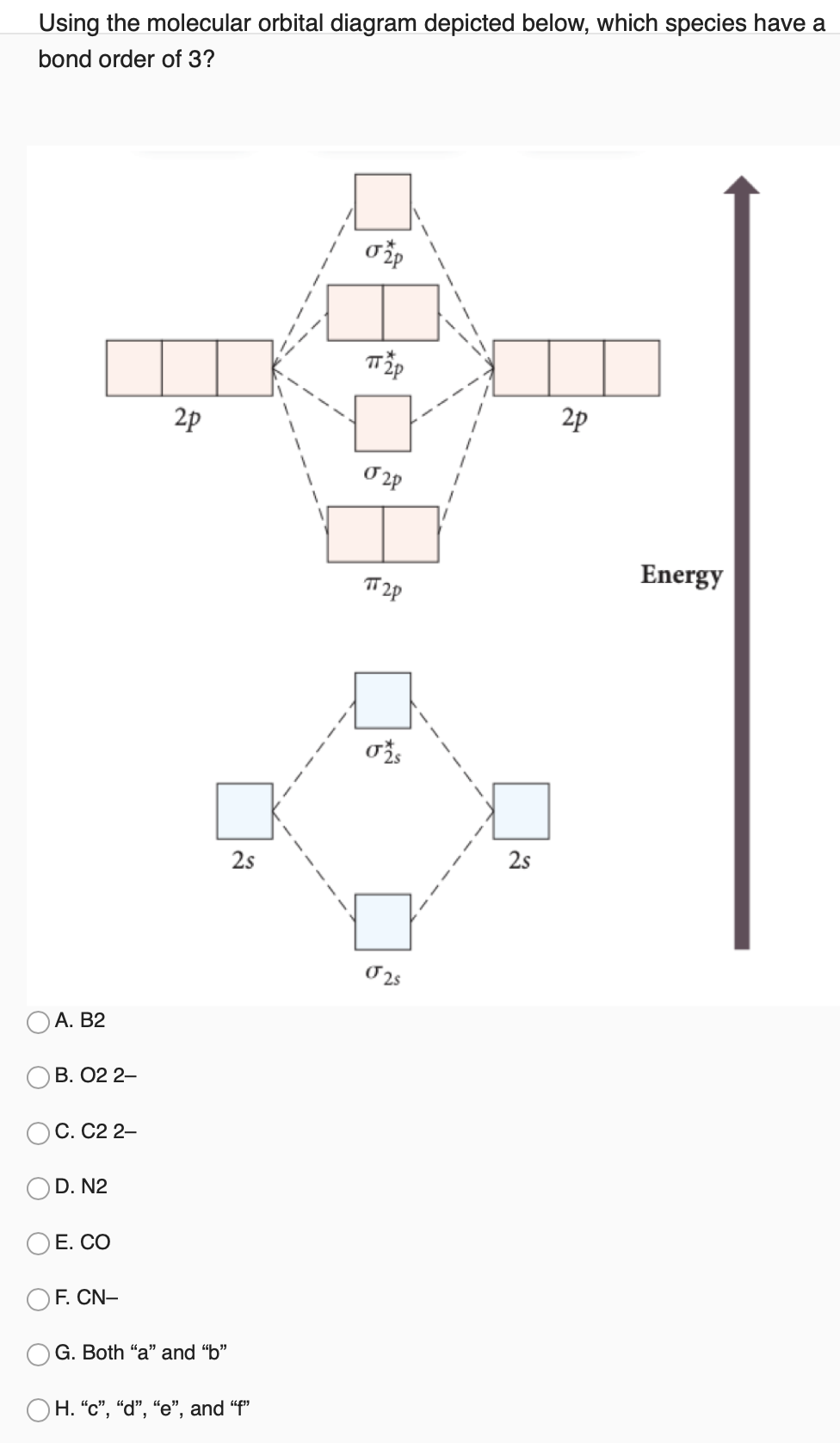
Sep 04, 2021 · A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the σ 1s bonding orbital; the electron configuration is \((σ_{1s})^2\). We represent this configuration by a molecular orbital energy diagram (Figure \(\PageIndex{8}\)) in which a single upward arrow indicates one electron in an orbital, and two (upward ... Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. Jan 15, 2019 · Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Jan 15, 2022 · A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory ... 29 Sept 2017 — Fluorine molecule is formed by the combination of atomic orbitals of two fluorine atoms, each having nine electrons, thus making 18 electrons.2 answers · 62 votes: O2 and F2 is an execption so their Z shell is down ...
In chemistry, Molecular orbital (MO) theory is a method for describing the electronic structure of molecules using quantum mechanics. Electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.Main postulates of this theory are Atomic orbitals of comparable Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Earth.It has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order 2 N b − N a = 2 8 − 4 = 2 Thus, oxygen molecule has … O2 Bond Order. Here are a number of highest rated O2 Bond Order pictures on internet. We identified it from reliable source. Its submitted by giving out in the best field. We receive this nice of O2 Bond Order graphic could possibly be the most trending topic as soon as we ration it in google gain or facebook.
Oxygen (1s 2 2s 2 2p 4), fluorine (1s 2 2s 2 2p 5), and neon (1s 2 2s 2 2p 6) then complete the already singly filled 2p orbitals; the last of these fills the second shell completely. Starting from element 11, sodium, there is no more space in the second shell, which from here on is a core shell just like the first. Thus the eleventh electron ...
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z …
Mar 18, 2018 · "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram.
Jan 28, 2022 · The molecular orbital diagram shows the energy state at each level where the excited state increases from the bottom to the top. The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule.

















0 Response to "34 o2 2- molecular orbital diagram"
Post a Comment