34 ethylene molecular orbital diagram
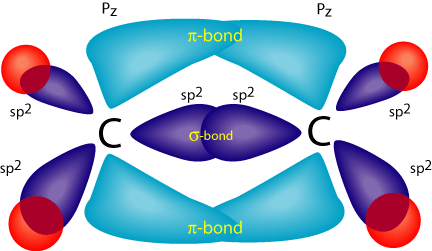
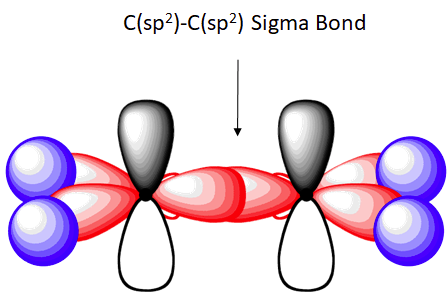
The hybrid orbitals are more prominent outward so that their ability to overlap is stronger than that of normal orbitals. Molecular Formula: A chemical formula is a brief way of expressing the number and type of atoms that make up a particular chemical compound. A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons.
Pi Molecular Orbitals of 1,3,5-Hexatriene. With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure. There are six adjacent carbon atoms involved in the pi ...
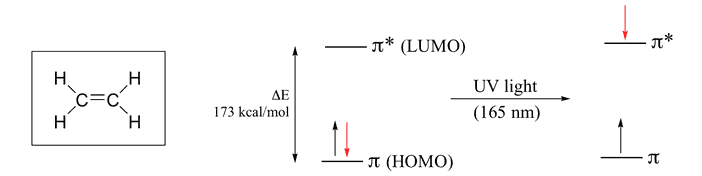
Ethylene molecular orbital diagram
a) Considering the molecular orbital diagram for ethylene above, draw the molecules (with some kind of three-dimensional representation) of the initial interaction of ethylene with a very strong acid (represent this as H-A). b) Finish the molecular orbital diagram of formaldehyde (H 2 CO) by making the molecular orbitals in the middle. Molecular Orbital Theory! While FMO theory allows prediction of reactions (by thermodynamics, regio or stereochemistry), all predictions seen so far have been qualitative! We have predicted that HOMO or LUMO levels raise or lower due to degree of mixing of orbitals and the charge, or electronegativity, of orbitals that are being mixed ! Orbital description of bonding between ethylene and a transition metal. This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon.
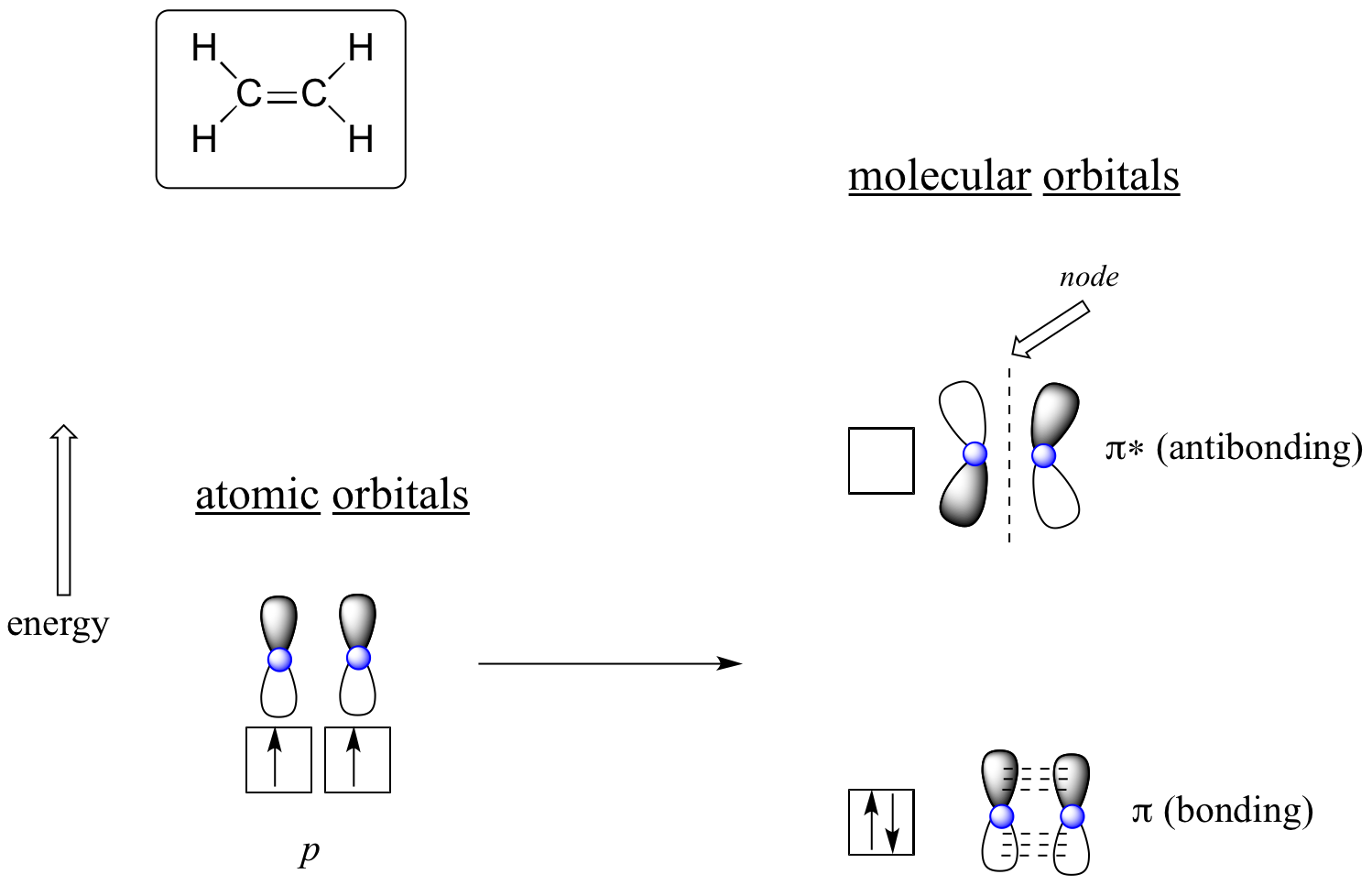
Ethylene molecular orbital diagram. In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals: ψ 1 and ψ 2 *, (also referred to as π 1 and π 2 *).. ψ 1 is a bonding molecular orbital, is occupied in the ground state, and is the Highest Occupied Molecular Orbital (HOMO). ψ 2 * is an antibonding molecular ... Molecular Orbitals: Ethene (Ethylene) Ethylene is the simplest molecule that has a double bond. As we saw from the valence bond model, we should find the presence of a σ-bond framework, and a π-bond between carbons. determine the shapes of the π molecular orbitals in ethylene. 6 Open the Hückel program. At the top of the window is a menu with the following options - File, Edit, View, Calculation, Help Below the menu is a tool bar. Below the tool bar is a page with a grid. Tool Bar The rate constant for the gas-phase reaction of ethylene with photochemically-produced hydroxyl radicals is 7.9X10-12 cu cm/molecule-sec at 25 °C (1). This corresponds to an atmospheric half-life of about 2 days at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm (2).
The unhybridized p-orbitals of the two carbon atoms overlap sidewise with each other to form weak pi bond. The bond consists of two electron clouds which lie above and below the plane of carbon and hydrogen atoms. (a) Formation of ethylene (b) Molecular orbital structure molecule of ethylene Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups. 14 molecular orbitals read in model 2. 0 molecular orbitals read. Mulliken charges found for Model 2. Molecular dipole for model 2 = {0, 0, 0} Time for openFile (./mo/ethylene.log): 101 ms. reading 12 atoms. ModelSet: haveSymmetry:false haveUnitcells:false haveFractionalCoord:false. 2 models in this collection. Chad introduces Pi Molecular Orbitals using Ethylene, drawing Bonding and Antibonding Molecular Orbitals and identifying the HOMO and LUMO.I've created an or...
This video shows that the pi framework and sigma framework of ethylene are distinct orbital sets. Molecular Orbitals: Example 1: Ethylene E E H2CCH2 p 2 sp2 1s p 2 sp2 1s σ σ* π π* A.O. A.O. C C HHC C C from other end of double bond A.O. means atomic orbitals (s, sp2, p) M.O. means molecular orbitals (σ , π ) C from left end of double bond M.O. Looking at both sigma and pi bonds Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. The above diagram shows the Molecular Orbital (MO) diagram of ethene/ethylene. Polarity of C2H4 The C2H4 molecule is non-polar in nature as all the atoms are symmetrically arranged across the molecule and both carbon atoms have the same influence on the bonded electrons.
Ethene: The simplest alkene is ethene. Its chemistry is dominated by two "frontier orbitals", that is the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO.
Molecular orbitals for ethene (ethylene) In the bonding pi orbital, the two shaded lobes of the p orbitals interact constructively with each other, as do the two unshaded lobes (remember, the arbitrary shading choice represents mathematical (+) and (-) signs for the mathematical wavefunction describing the orbital).

This photograph depicted Enteric Diseases Laboratory Branch (EDLB), Public Health scientists, as they were preparing enteric bacteria samples for “DNA fingerprintingâ€, using pulsed-field gel electrophoresis (PFGE).
Ethene is built from hydrogen atoms (1s 1) and carbon atoms (1s 2 2s 2 2p x 1 2p y 1). A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p.
Molecular Orbitals in Conjugated Systems. According to the frontier orbital theory, the chemistry of conjugated π systems is largely determined by the HOMO and LUMO π orbitals in the reactant molecules.The outcome of reactions involving interaction of π orbitals can be rationalized using the concepts of orbital phase and orbital symmetry.
Bonding orbitals in Ethylene (Ethene) sp. 2. 1 model in this collection. Use getProperty "modelInfo" or getProperty "auxiliaryInfo" to inspect them. Use "set autoLoadOrientation TRUE" before loading or "restore orientation DEFAULT" after loading to view this orientation. spinFPS is set too fast (30) -- can't keep up!
Molecular orbital energy level diagram for the two-ethylene system, before the molecules have come into contact, plotted with respect to the level degeneracy. The HOMO level has been set to the ...
occupied molecular orbitals and N/2 unoccupied ones. For the ground state, we of course occupy the lowest energy orbitals. ... Hückel theory for ethylene, we find that a single ethylene double bond has an energy EC=C = 2α+ 2β Thus, if benzene simply had three double bonds, we would expect it to have a total energy of
Molecular Orbital (MO) Theory (continued 1) • Filling of MOs with electrons is governed by the same rules as for atomic orbitals • Aufbau principle - Fill MOs beginning with the lowest energy unoccupied molecular orbital • Pauli exclusion principle - No more than two electrons can be accommodated in a MO, and their spins must be paired
In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles. C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o.

Vaccine. Dr. J. Michael Hamilton preparing the carcinoembryonic antigen (CEA) vaccinia vaccine used to try to prevent cancer. He is diluting the concentrated vaccinia virus into a dose level appropriate for administration to a patient. This vaccinia marks any cancer cells expressing the CEA.
A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons.
Orbital description of bonding between ethylene and a transition metal. This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon.
Molecular Orbital Theory! While FMO theory allows prediction of reactions (by thermodynamics, regio or stereochemistry), all predictions seen so far have been qualitative! We have predicted that HOMO or LUMO levels raise or lower due to degree of mixing of orbitals and the charge, or electronegativity, of orbitals that are being mixed !
a) Considering the molecular orbital diagram for ethylene above, draw the molecules (with some kind of three-dimensional representation) of the initial interaction of ethylene with a very strong acid (represent this as H-A). b) Finish the molecular orbital diagram of formaldehyde (H 2 CO) by making the molecular orbitals in the middle.
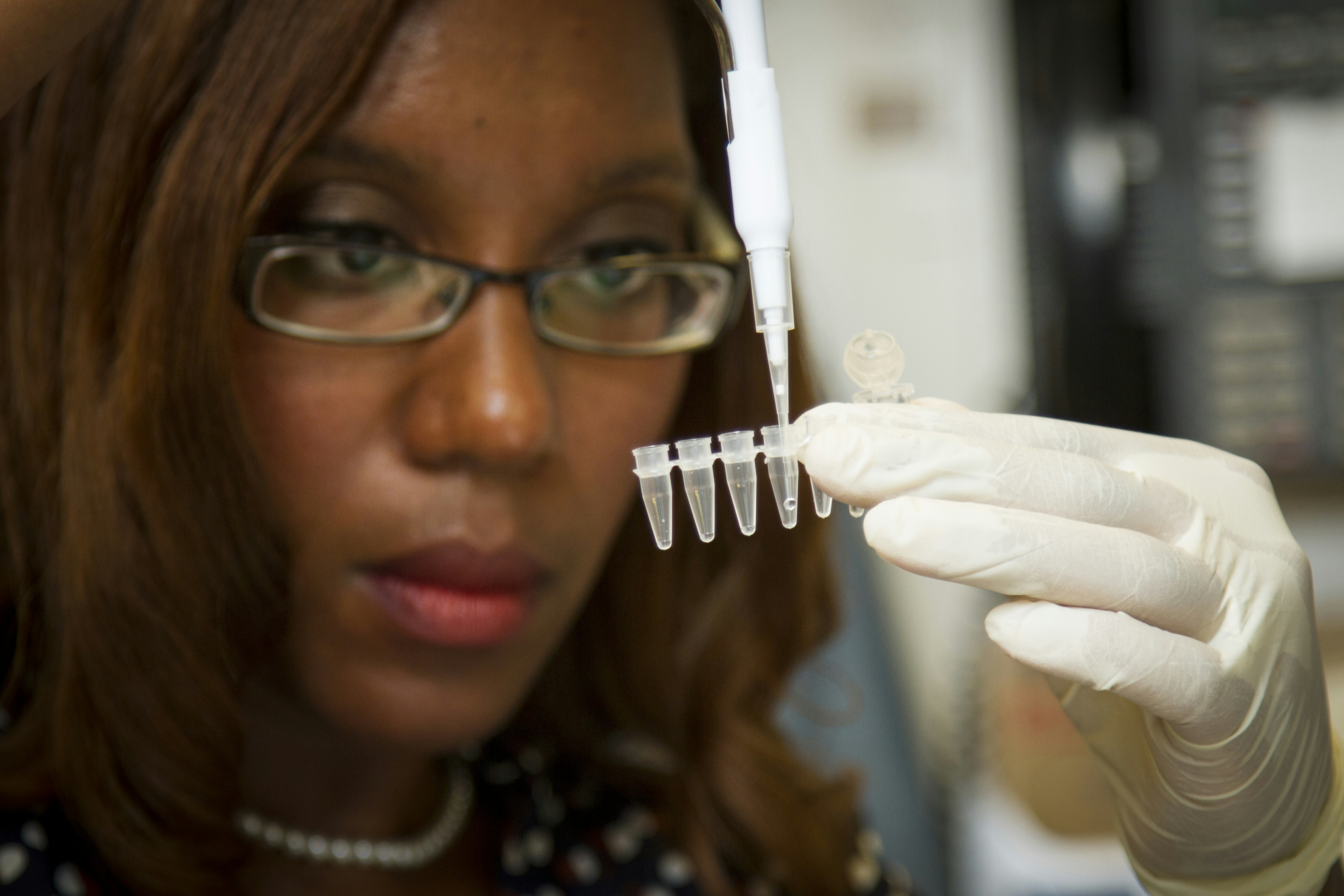
Chanelle Case Borden, Ph.D., a postdoctoral fellow in the National Cancer Institute's Experimental Immunology Branch, pipetting DNA samples into a tube for polymerase chain reaction, or PCR, a laboratory technique used to make multiple copies of a segment of DNA.

3rd Meeting of the Science Demonstration Conference. Dr. Margaret Kelly, Medicine Branch, "Chemical Carcinogenesis in Newborn Animals". 1962

The National Cancer Institute's Natural Products Branch at the Frederick National Laboratory for Cancer Research is the largest program to collect materials worldwide from marine, plant, and microbial sources so they may be studied for possible medical uses. The fermentation lab grows fungal and bacterial cultures in liquid media. These materials are extracted using organic solvents, and eventually tested in the NCI cancer screens.







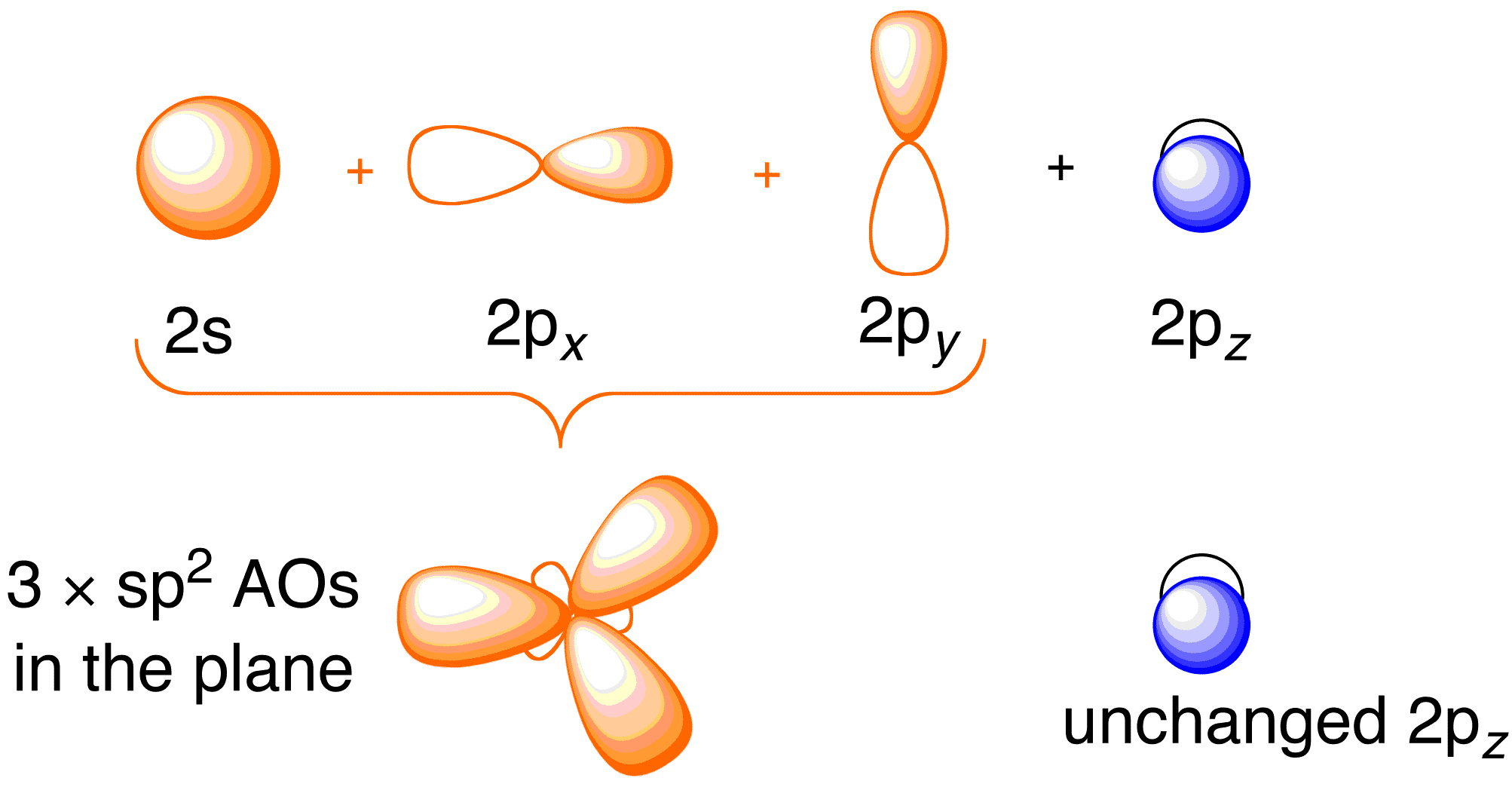







0 Response to "34 ethylene molecular orbital diagram"
Post a Comment