39 orbital diagram for silver
From the periodic table we know that the at atomic number of silver is 47 so it will have the electronic configuration of [Kr]5s14d10 this happens in the ...1 answer · Top answer: Hint: According to Dalton’s theory the atom is the most basic form of matter but the atom contains various subatomic particles like the electrons and ...
Write the observed electron configuration of Ag. Express your answer in complete form in order of orbital filling. For example, 1s2 2s2 should be entered as ...1 answer · Top answer: Ag has an atomic number of 47 which means it has 47 electrons that would be used to fill the orbitals.Recall:s orbital → can hold a maximum of 2 electronsp ...
Well, we can write the electronic configuration of the silver atom... A quick glance at the Periodic Table tells us that Z_"Ag"=47..and so for the NEUTRAL atom.... We use the Aufbau process to write the electronic distribution of the SILVER atom... underbrace(1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)3d^(10)4s^(2)4p^(6))_"electronic configuration of krypton"5s^(1)4d^(10) That the configuration shows 5s^(1 ...
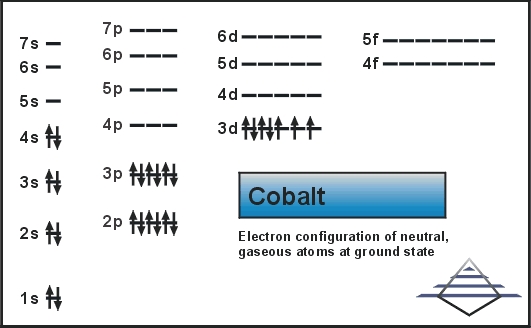
Orbital diagram for silver
Silver is a chemical element with the symbol Ag (from the Latin argentum, derived from the Proto-Indo-European h₂erǵ: "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. The metal is found in the Earth's crust in the pure, free elemental form ("native silver ...
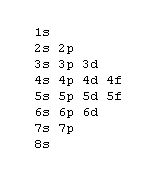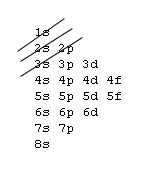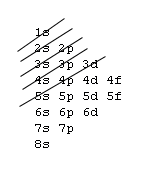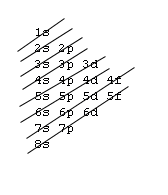
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
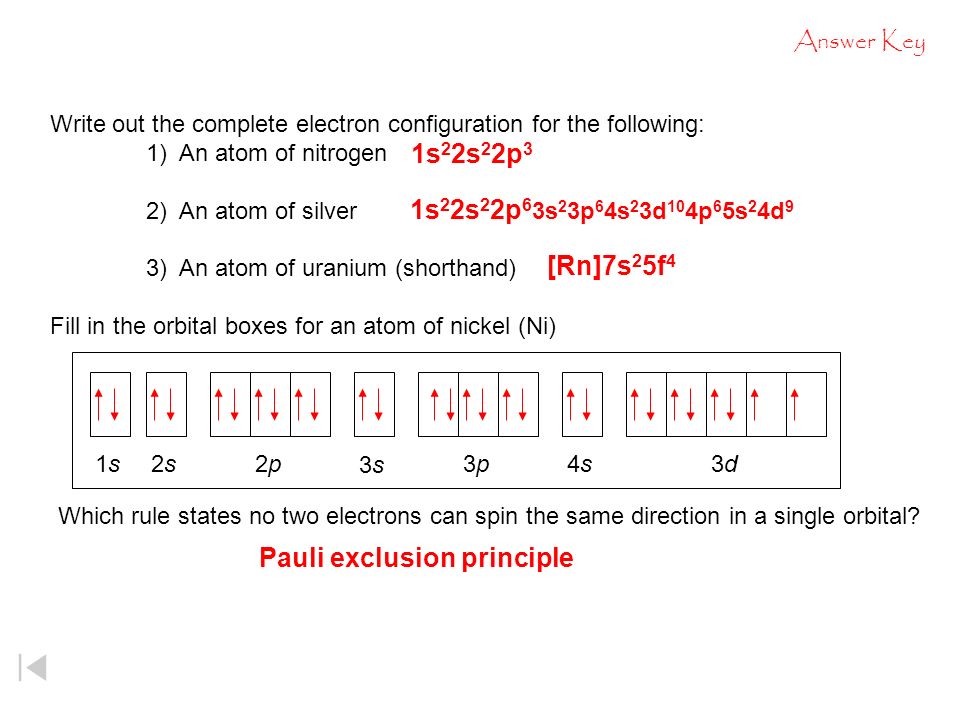
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Orbital diagram for silver.
For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The [latex]n = 1[/latex] shell is completely filled in a helium atom.
Noble Gas Notation: [Rn] 7s² Four quantum numbers for last electron in orbital diagram: 1. n=7 2. Since fully filled orbital are more stable than half filled the vanadium will have 2 electrons in 4s orbital. (4) Electron affinity for d-orbital completion increases with increasing period number, n.
The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ...
5 Dec 2020 — Silver Electron Configuration (Ag) with Orbital Diagram ... Electron Configuration For Silver: Silver is a chemical element that has a chemical ...
Sources of Silver: Found in ores called argentite (AgS), light ruby silver (Ag 3 AsS 3), dark ruby silver (Ag 3 SbS 3) and brittle silver. Silver is often obtained as a by-product of refining other metals like copper and gold. World wide production is around 9950 tons per year. Primary mining areas are Mexico, Bolivia, Honduras, Canada, USA ...
Silver-Line SL-1218R Orbital Floor Refinisher Owners Manual Thank you for purchasing a SL-1218R floor refinisher. When used properly this unit will perform well as a rental machine. Please review and follow the suggested procedures for operation of this machine.
2. Electron Configuration (quicker to draw than orbital filling diagrams) Ex. O2 1s2 2s2 2p4. 3. Electron Dot shows only the valence (outer energy level) electrons. . Ex. Oxygen atom . O :. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams . for the following elements. Table: Element Orbital Filling Diagram
An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
Silver (Ag) has an atomic mass of 47. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
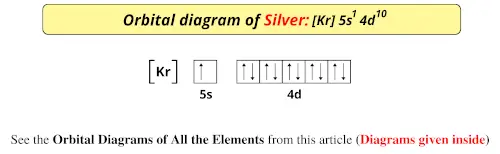
What is the electron configuration of Ag+? Answer: Silver (Ag) has an electron configuration of [Kr] 4d105s1. The element is much more stable and has a lower energy when the 4d orbital is filled, so one electron is placed there, rather than in the 5s orbital.
1 answerTo create the orbital configuration, we can refer to the orbital diagram below. As silver has an atomic number of 47, a neutral atom has 47 protons...
There is 1 s orbital, 5 p orbitals, 10 d orbitals and so on. This numbers points to one of the orbitals. ms - spin quantum number, represents the spin of an electron. Values +1/2 and -1/2. Back to your question - quantum number of outermost s orbital of silver. Silver has an electron configuration [Kr] 4d 10 5s 1.
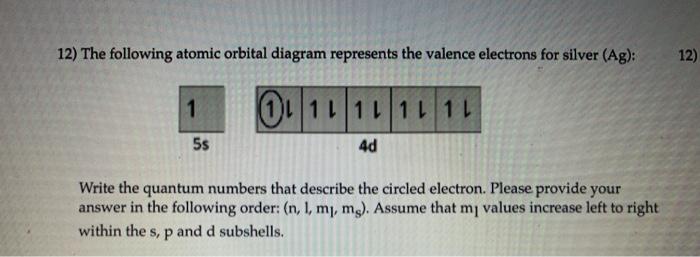
7. (8 pts.) Construct an electron configuration and orbital diagram for the element silver (Ag). For the electron configuration you may use the noble gas shorthand representation if you wish. Question: 7. (8 pts.) Construct an electron configuration and orbital diagram for the element silver (Ag).
Download scientific diagram | 2 Orbital diagram. ... Silver, Chemistry and Catalysis | ResearchGate, the professional network for scientists.
Silver Ag has the atomic number 47. It is a soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, ...4 answers · 14 votes: See, silver has atomic no. 47… So, k shell or orbital can have only 2 electrons ...
2. Electron Configuration (quicker to draw than orbital filling diagrams) 2 2 Ex. O 2 1s 2s 2p4 3. Electron Dot shows only the valence (outer energy level) electrons . . Ex. :Oxygen atom . O . 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram
6. Draw the orbital energy (i.e., box) diagram for the electrons beyond the [Kr) core of the element silver (Ag). If all the electrons are paired in the orbital energy diagram, the element is said to be diamagnetic, meaning that atoms of the element will interact weakly with an external magnetic field.
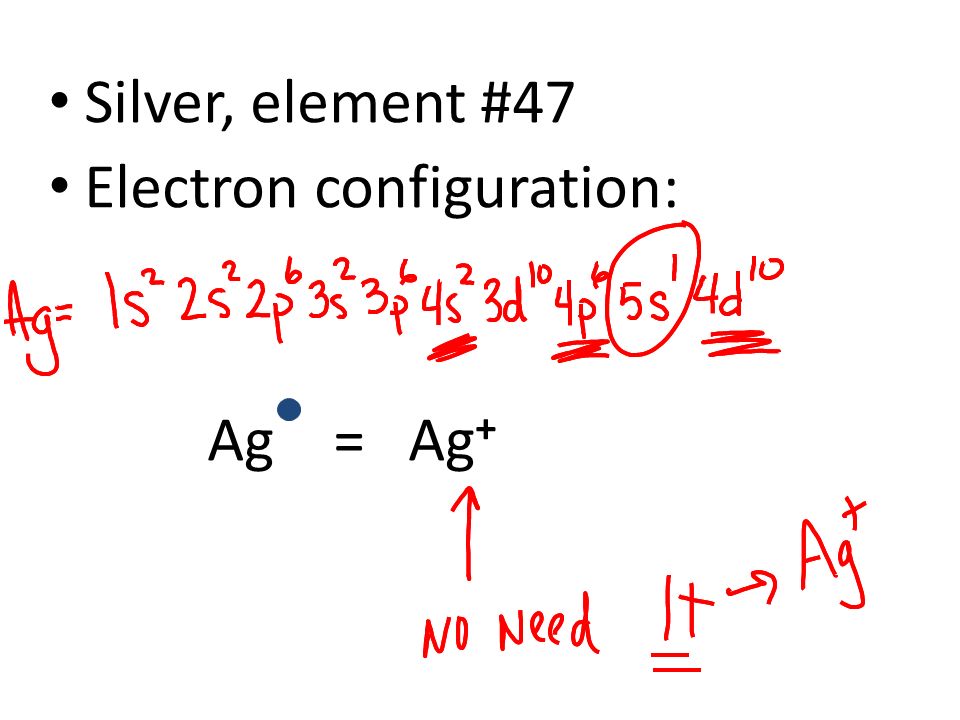
Ag, or silver, has the atomic number 47. Its orbital notation is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
The thing to remember here is that in silver's case, the 4d orbitals will be completely filled. That implies that you won't have two electrons in the 5s orbital, since one will be kept in the lower 4d orbitals. This means that the electron configuration of silver will be Ag: 1s22s22sp63s23p63d104s24p64d105s1
Electron configuration is how the electrons are distributed among the various atomic orbitals in an atom. Only two electron can be located in each orbital. The "s" sublevel contains 1 orbital, the ...
Ag (Silver) is an element with position number 47 in the periodic table. Located in the V period. Melting point: 961.9 ℃. Density: 10.49 g/cm 3 . The order of filling the orbitals with electrons in the Ag atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9.
Silver is a relatively soft, shiny metal. It tarnishes slowly in air as sulfur compounds react with the surface forming black silver sulfide. Uses. Sterling silver contains 92.5% silver. The rest is copper or some other metal. It is used for jewellery and silver tableware, where appearance is important.
The orbital diagram for hydrogen can be represented in the following way. This notation uses a box to represent the orbital, the label for the orbital and an arrow to represent the electron. The electronic configuration for hydrogen can be written as 1s 1. This is a short-hand notation which identifies the level, the sublevel and the number of ...
Orbital diagram of Silver (Ag) 48: Orbital diagram of Cadmium (Cd) 49: Orbital diagram of Indium (In) 50: Orbital diagram of Tin (Sn) 51: Orbital diagram of Antimony (Sb) 52: Orbital diagram of Tellurium (Te) 53: Orbital diagram of Iodine (I) 54: Orbital diagram of Xenon (Xe) 55: Orbital diagram of Caesium (Cs) 56: Orbital diagram of Barium (Ba ...












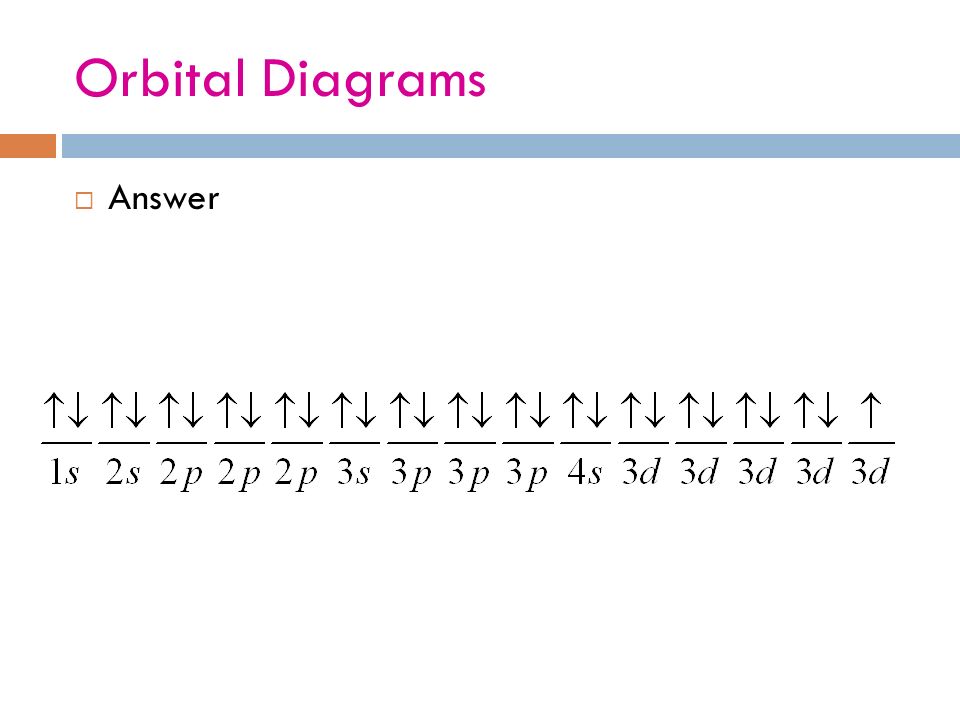


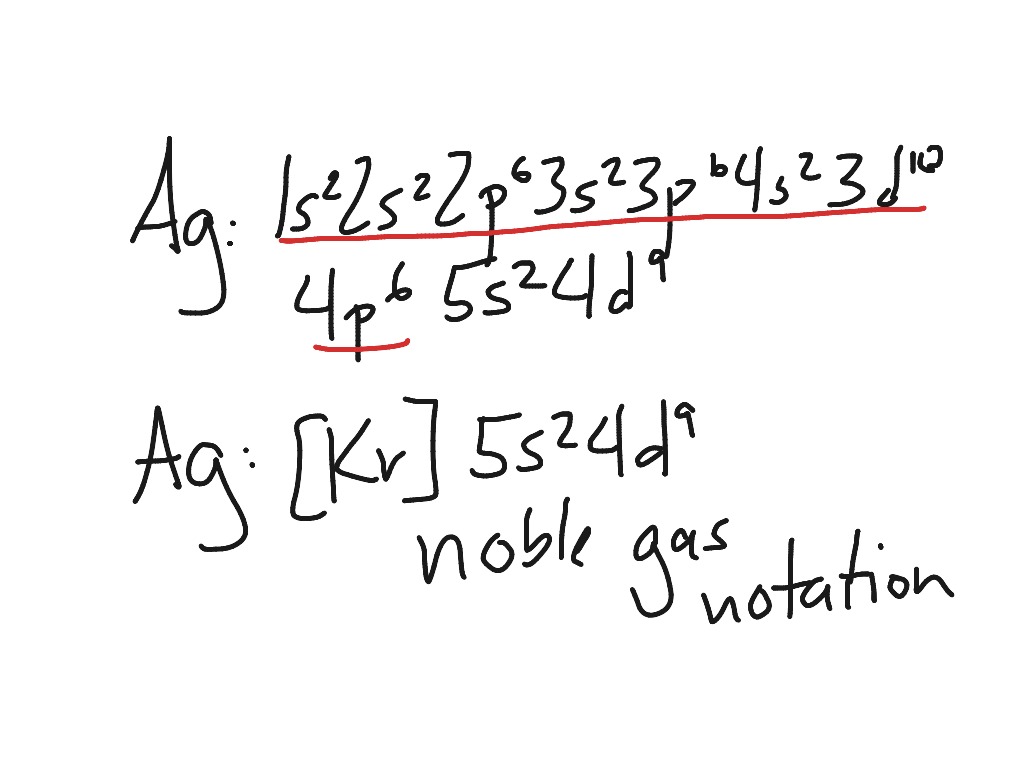









0 Response to "39 orbital diagram for silver"
Post a Comment