38 v5+ orbital diagram
Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom.
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system ...
Molecular orbital diagram practice worksheet. For the following elements. the four orbitals as bonding, non-bonding or antibonding. 1 and inserting your answers in the answer blanks. Nitrogen is the seventh element with a total of 7 electrons. 30 M solution of NaCl (58. 69 m 50. The HR Diagram. Molecular Orbital Worksheet 1. 54 m C) 17.

V5+ orbital diagram
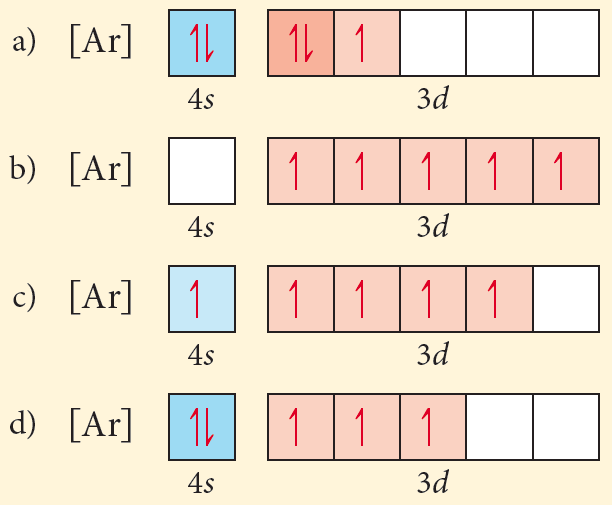
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ...
About SanderParts.com. We are a leading provider of hardwood flooring tools, products and equipment at the LOWEST PRICES you won't find anywhere else. Quality finishes, abrasives, equipment and supplies keep contractors smiling in their flooring jobs and their bank accounts! Make SanderParts.com your choice for all your hardwood floor care needs.
To determine whether the elements are paramagnetic or diamagnetic, write out the electron configuration for each element. He: 1s 2 subshell is filled. Be: 1s 2 2s 2 subshell is filled. Li: 1s 2 2s 1 subshell is not filled. N: 1s 2 2s 2 2p 3 subshell is not filled.
V5+ orbital diagram.
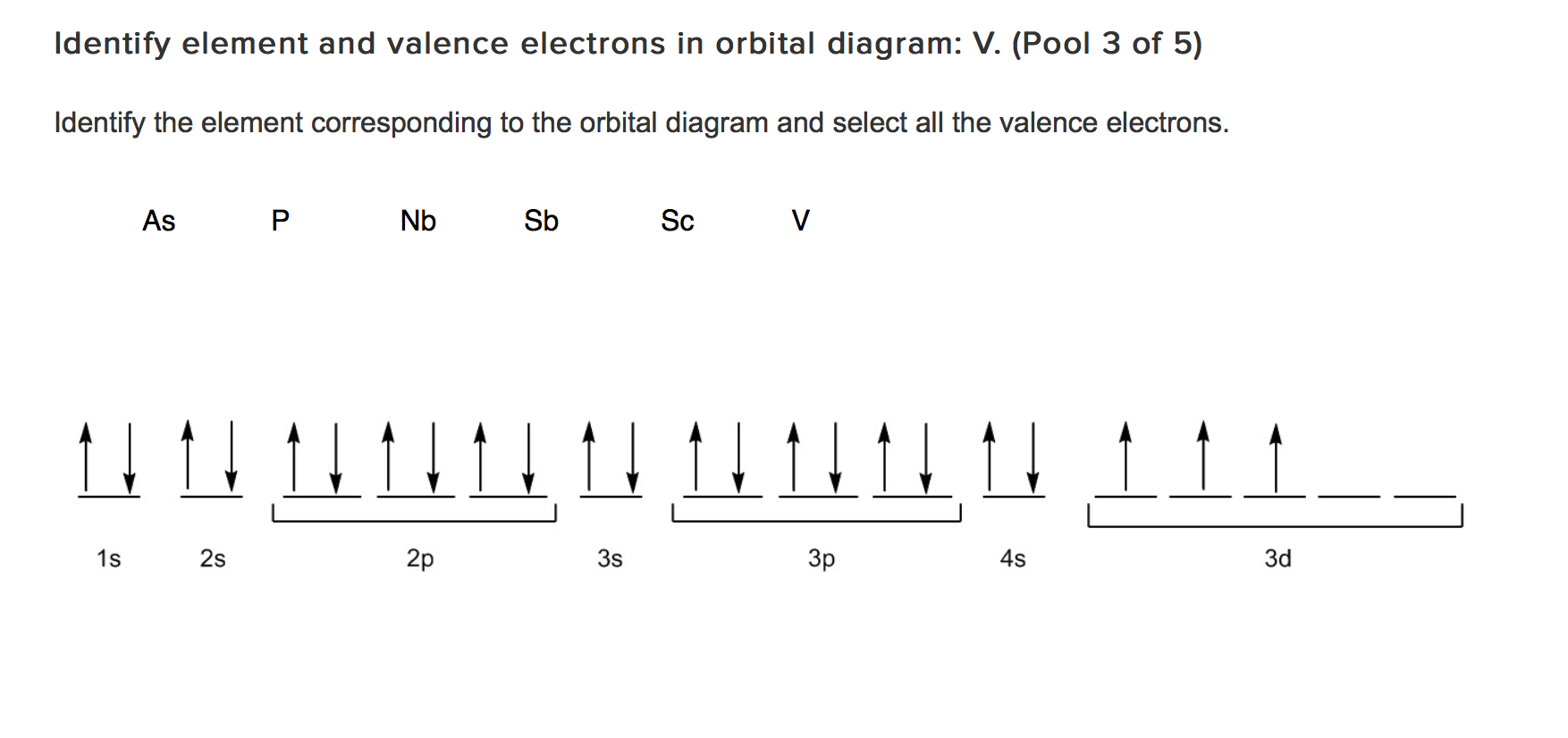
What element is represented by this orbital diagram? Pauli Exclusion Principle. 2 electrons in the same orbital must have opposite spins. Hund's Rule. Electrons don't pair up in orbitals of equal energy until they have to, and all electrons in singly occupied orbitals have the same spin.
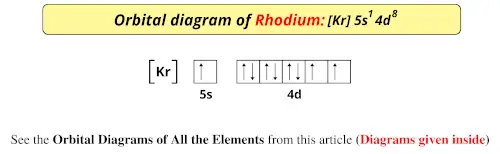
Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the orbitals are represented by half arrows.
Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.
To write the configuration for the Vanadium and the Vanadium ion, first we need to write the electron configuration for just Vanadium (V). We first need to ...
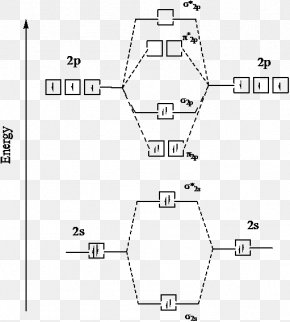
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mo stly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine The energy curves for ψ + and ψ-reveal the following properties of the ion H 2 +.
Chemistry Q&A Library Write orbital diagram for V5+ Write orbital diagram for V5+ close. Start your trial now! First week only $4.99! arrow_forward. Question. Write orbital diagram for V 5+ check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here.
Dec 19, 2018 · V5+ Orbital Diagram. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. can be accommodated in the metal d orbitals. • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions . σ-ML4 Tetrahedral MO Diagram e.
Calculating the Energy Level of an Orbital. In a single electron, Hydrogen-like atom, the orbital energy i.e. the energy of that one electron depends just on the principal quantum number (n). In orbitals chemistry when it comes to filling up the atom with electrons, the Aufbau principle tells the lower energy level orbitals always come first.
First, let's look at those for the neutral atom, and then work our way to the cation. VANADIUM (V) "V" is atomic number 23, so its configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^3 4s^2. In shorthand it is [Ar] 3d^3 4s^2. This is an expected configuration; not an oddball element. Since the 4s orbital is higher in energy, its electrons will be removed first.
Feb 18, 2021 · Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration February 18, 2021 by Sneha Leave a Comment Vanadium Electron Configuration : When it comes to electronic configuration, it is one of the major topics in chemistry as we have mentioned before in our article.
Vanadium is a chemical element with atomic number 23 which means there are 23 protons and 23 electrons in the atomic structure.The chemical symbol for Vanadium is V. Electron Configuration and Oxidation States of Vanadium. Electron configuration of Vanadium is [Ar] 3d3 4s2. Possible oxidation states are +2,3,4,5. Electron Configuration
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium.
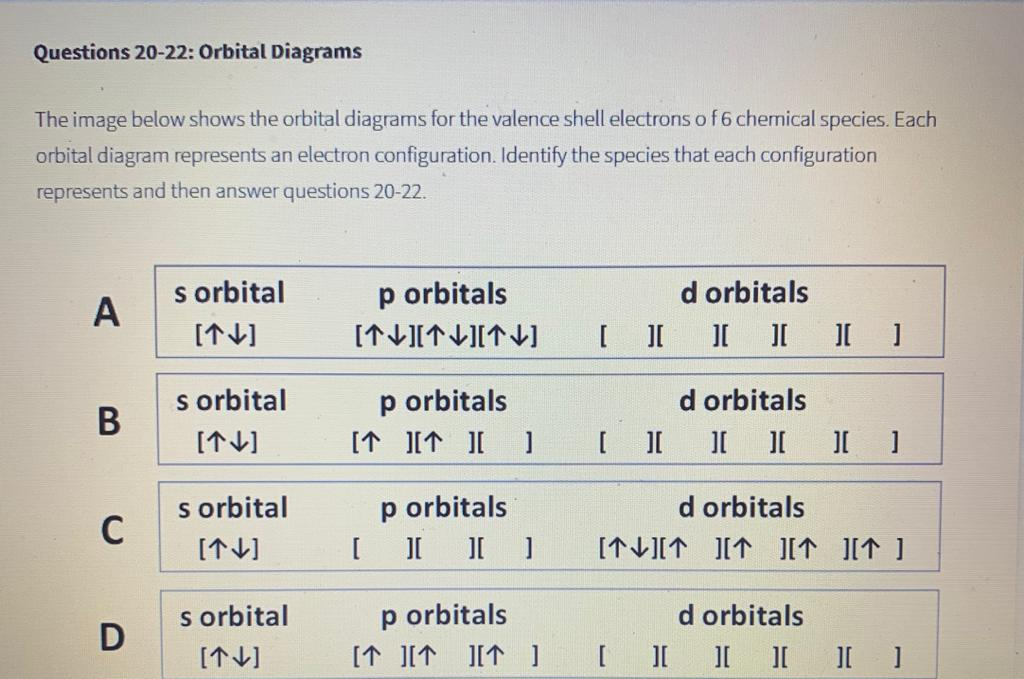
Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled Reset Help P 111 1s 25 38 4s 2p 3p 4p 3d 61 G1 G1 GIG1 G1 GIG1 G1 G1 G1G1G1 G1|| G1 G16161 62 G2 G2 G2 G2 G2 G2 G2 Submit Request Answer Part B Enter an orbital diagram for Cr3 Drag the ...
An orbital diagram naturally leads to the writing of an electron configuration. V5 + Cr3+ Ni2+ Fe3+ Explore each Elements orbitals and electron configuration. It is a powerful fluorinating as well as an oxidizing agent. For each electron shell atom diagram, the element symbol is listed in the nucleus.
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+
Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In
Molecular orbital diagram practice worksheet. For the following elements. the four orbitals as bonding, non-bonding or antibonding. 1 and inserting your answers in the answer blanks. Nitrogen is the seventh element with a total of 7 electrons. 30 M solution of NaCl (58. 69 m 50. The HR Diagram. Molecular Orbital Worksheet 1. 54 m C) 17.
Q. Write orbital diagrams for each of these ions.V5+ Q. Write orbital diagram to represent the electron configurations-without hybridization-for F in SF2. Q. Choose the correct orbital diagram for vanadium. See all problems in The Electron Configuration: Ions Frequently Asked Questions What scientific concept do you need to know in order to ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Nov 25, 2021 · The orbital diagram would show the spin of each electron in the electronic orbital as well as identify the type of orbital being occupied by the electrons. Answer and Explanation: 1. See the answer See the answer done loading. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+. Determine if the ion is diamagnetic or paramagnetic.
Found in the minerals patronite (VS4), vanadinite [Pb 5 (VO 4) 3 Cl], and carnotite [K 2 (UO 2) 2 (VO 4) 2 .3H 2 O]. Vanadium is usually produced as a by-product of refining other ores and from Venezuelan oils. Annual world wide production is around 7,000 tons. Uses of Vanadium:
AHRIMy Essay Gram - We are your custom essay writing service Newton S Laws of Motion Questions and Answers | Study.comMolecular orbital diagram practice worksheetCCNA 1 Final Exam Answers 2019 (v5.1+v6.0) Introduction to Vectors and Projectiles Review - with Answers #21D Kinematics Review
9. Electrons in an orbital with l = 3 are in a/an. A) d orbital. B) f orbital. C) g orbital. D) p orbital. E) s orbital. 10. The number of orbitals in a d subshell is. A) 1 B) 2 C) 3 D) 5 E) 7. 11. "No two electrons in an atom can have the same four quantum numbers" is a statement of. A) the Pauli exclusion principle. D) de Broglie's relation.






















![Solved The correct orbital diagram for Pb [Xe] a) [Xe ...](https://media.cheggcdn.com/media%2Fa91%2Fa9100cd2-e53a-44f2-b0ef-c4e0a304bbc2%2Fimage)

0 Response to "38 v5+ orbital diagram"
Post a Comment