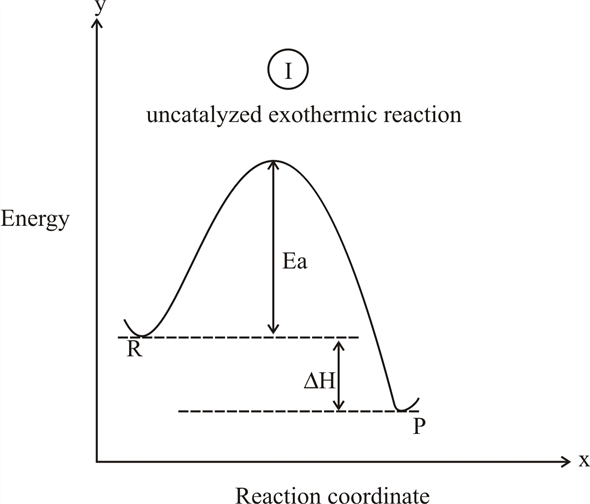
38 reaction coordinate diagram exothermic
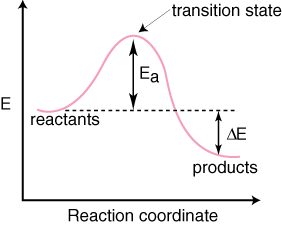
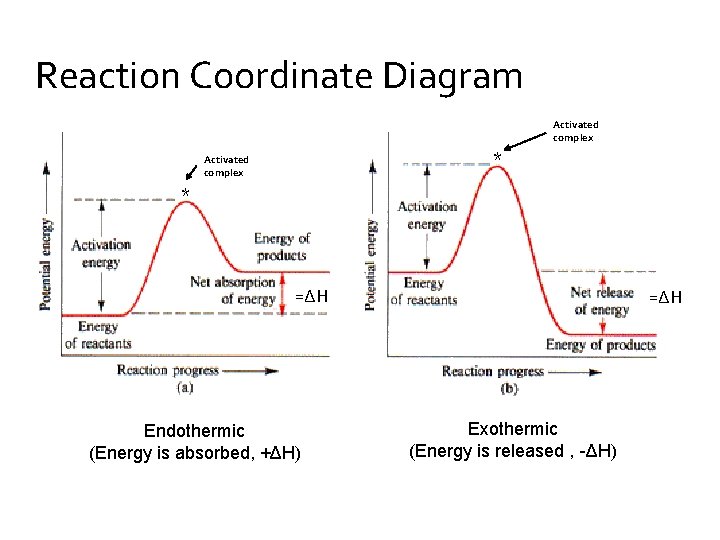
The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. A Draw a reaction coordinate diagram that is consistent with ...
Exothermic Reaction Coordinate Diagram; Endo/Exothermic Reactions - VISTA HEIGHTS 8TH GRADE SCIENCE; Solved: Exothermic reactions are usually self-sustaining ... Exothermic Reaction (3 of 3) - Stock Image - C002/8012 ... Endothermic and Exothermic reactions - YouTube Exothermic - Key Stage Wiki ...
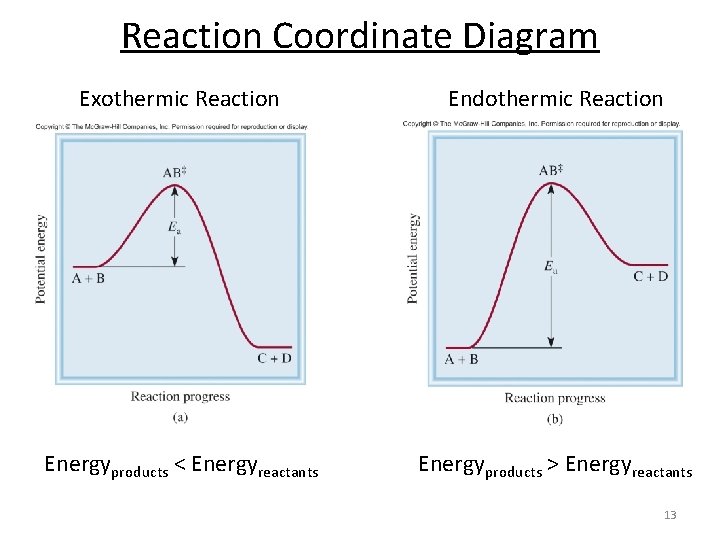
Figure 18.4. 1: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and Δ H is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the ...

Reaction coordinate diagram exothermic
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction .
In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, ...
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction.
Reaction coordinate diagram exothermic.
The given diagram is called a reaction coordinate diagram. In this, the energy of species is plotted as a function of reaction progress. It represents an exothermic reaction. For example, respiration is an exothermic process. Respiration is defined as the oxidation of food to release energy. Since energy is released, it is an exothermic process.
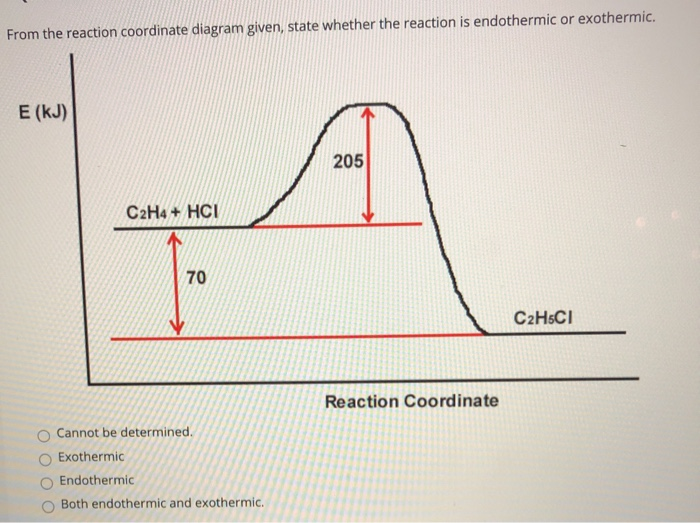
This chemistry video tutorial focuses on potential energy diagram s for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... The potential energy diagram and balanced equation shown below represent a reaction between solid carbon and hydrogen gas to produce 1 mole of C2H4(g) at 101.3 kPa and 298 K. Identify one change in the reaction conditions, other than ...
However, if I were to draw an energy/reaction coordinate diagram for this mechanism, the more reactive protonated alcohol would be higher up in energy than the unprotonated alcohol so I'm not sure if the initial protonation step would be exothermic or endothermic. This problem's been plaguing me for days now.
Draw An Enthalpy Diagram For A General Exothermic Reaction Label The Axis Reactants Products And Del. Write A Balanced Equation And Draw An Enth Clutch Prep. Topic 5 1 Exothermic And Endothermic Reactions Heat And. Label Free Detection Techniques For Protein Microarrays. Calorimetry Chemistry 2e.
What is a reaction coordinate diagram? It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint.
Energy Diagrams, Activation Energies, Gibbs Free Energy Change The Gibbs free energy (∆G) of a reaction is a measure of the thermodynamic driving force that makes a reaction occur. A negative value for ∆G indicates that a reaction can proceed spontaneously without external inputs, while a positive value indicates that it will not.
The " reaction coordinate" plotted along the abscissa represents the diagram s can describe both exothermic and endothermic reaction s. A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is ...
An exothermic reaction is one where at the end of the reaction, energy is released into the surroundings. Most reactions are exothermic, because in order to ...
A chemical reaction in which heat is absorbed is called án endothermic reaction. What is energy profile diagram? For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products.
36 free body diagram of block on incline Written By Robert T. Arbuckle Friday, December 3, 2021 Add Comment Edit 2. The normal force: The block is sitting on the incline.So the block exerts a force on the surface of the incline.So from Newt on 's Third Law (acti on-reacti on), the incline exerts a force back on the block.This force, called the 'Normal force', acts on the block so it must be ...
Answer (1 of 2): Some you can feel. Others you need an elaborate setup. Felling method: Do the reaction in a test tube and feel the outside of the test tube. If it s getting warm, the reaction is liberating heat, that is exothermic reaction. If it is cooling then the reaction is endothermic. Elab...
Answer (1 of 2): The rates of all reactions will increase with temperature. This is due to the fact that reactions require collisions and/or may have an activation barrier. Everything else describes the effect of temperature on the equilibrium constant.
Sketch a detailed potential energy diagram for this exothermic reaction. Make sure you show all species in the reaction coordinate diagram, the activation energies and the transition state energy ...
12+ Reaction Energy Diagram. An activation energy diagram with reactant energy lower than product energy, i.e. For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products.
35 cub cadet lt1050 belt diagram; 37 reaction coordinate diagram exothermic; 35 2003 ford taurus spark plug wiring diagram; 35 2001 nissan altima belt diagram; 36 well water storage tank diagram; 37 co2 molecular orbital diagram; 37 kinetic friction free body diagram; 36 smith and wesson m&p 15 sport parts diagram; 38 daiwa reel parts diagram
This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
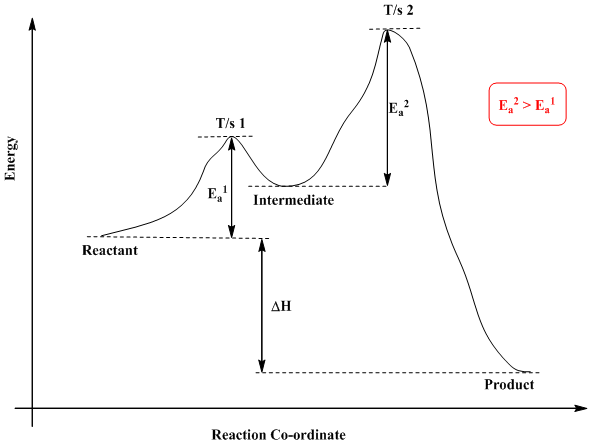
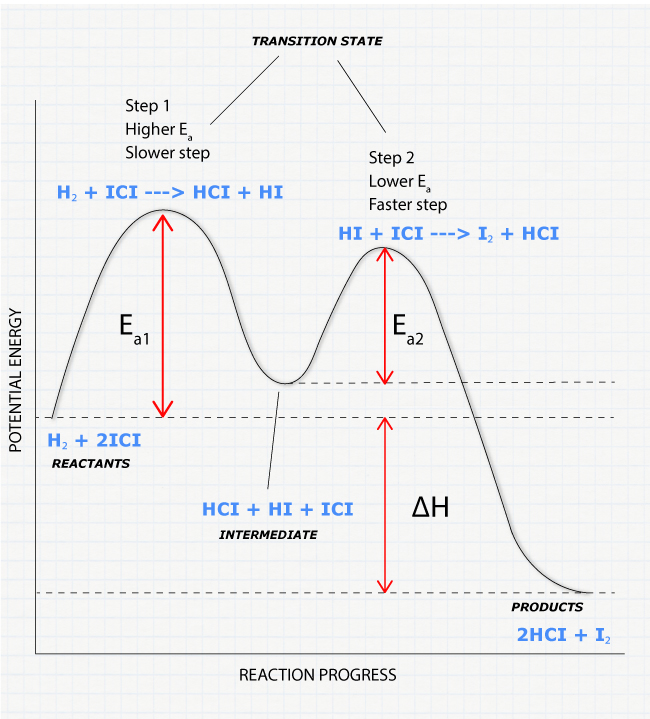
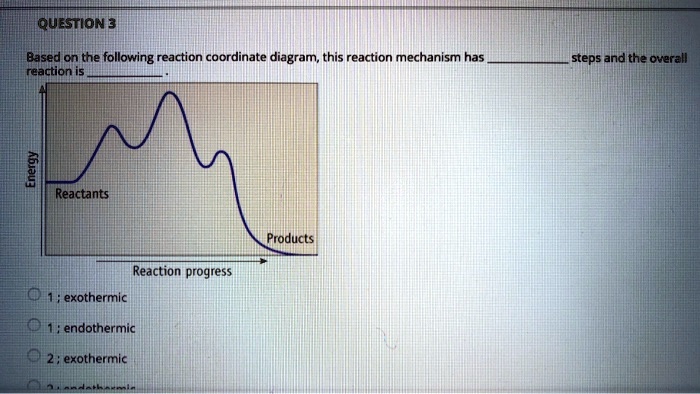
Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states.
Exothermic reactions with negative delta H values are favorable. However, when delta S is a positive value, that is favorable because it indicates an increase in entropy. So, when there is both a positive delta H and a positive delta S, the reaction is spontaneous (has a negative delta G) at very high temperatures.
If the reaction is exothermic, then adding heat will make it favor the reverse reaction, which is endothermic and so will use up the heat (decreasing K). Also, remember a higher K means products are favored and a small K means reactants are favored because of the way K is set up as a fraction with products on top and reactants on the bottom.
Stack Exchange network consists of 178 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.. Visit Stack Exchange
exothermic reaction专题🌟整理关于💖相关图片资讯希望大家喜欢。 Endo/Exothermic Reactions - VISTA HEIGHTS 8TH GRADE SCIENCE
The reaction is a single step reversible reaction. The energy of activation for the forward reaction is 28.9 kJ and that for the backward reaction is 41.8 kJ. Draw energy level diagram for the reaction.
Oct 17, 2019 — You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, ...
The highest point on the diagram is the activation energy E a the energy barrier that must be overcome for a reaction to occur. TextO_2textg textN_2textg 2textNO g Notice that the activation energy for the endothermic reaction is much greater than for the exothermic reaction.
Reaction Coordinate - A B - C 0 energy. Physical Science Worksheets and Printables. Is the reaction in 6 exothermic or endothermic. Complete Program Department Of Mathematics And Computer . Energy Quiz Pe Ke Roller Coasters Pendulum Energy Science Lessons Energy Quiz Pendulum . Http Irondale Weebly Com Uploads 2 4 2 5 24252776 Rxn Diagram ...
Elementary Reaction (one step) Two Step Reaction \[ \text{Reactants} \rightarrow \text{Products} \nonumber\] This is a sample reaction coordinate of an elementary reaction. Note that there is one transition state and no intermediate. Elementary steps cannot be broken down into simpler reactions.
Energy Diagram. Q. Draw a rough sketch of the energy profile for each of the following cases:b. ΔE = -10 kJ/mol, Ea = 50 kJ/mol. Solved • Nov 7, 2018. Energy Diagram. Q. The activation energy for some reaction X2 (g) + Y2 (g) → 2XY (g)is 167 kJ/mol, and ΔE for the reaction is 128 kJ/mol. What is the activation energ...

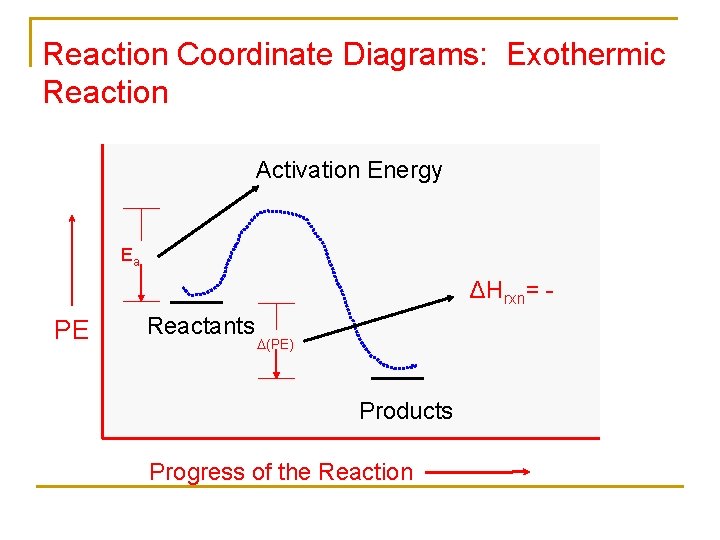
Which reaction coordinate diagram represents a reaction in which the activation energy, ea, is 50 kj•mol –1 and the δhrxn is –15 kj•mol –1?























0 Response to "38 reaction coordinate diagram exothermic"
Post a Comment