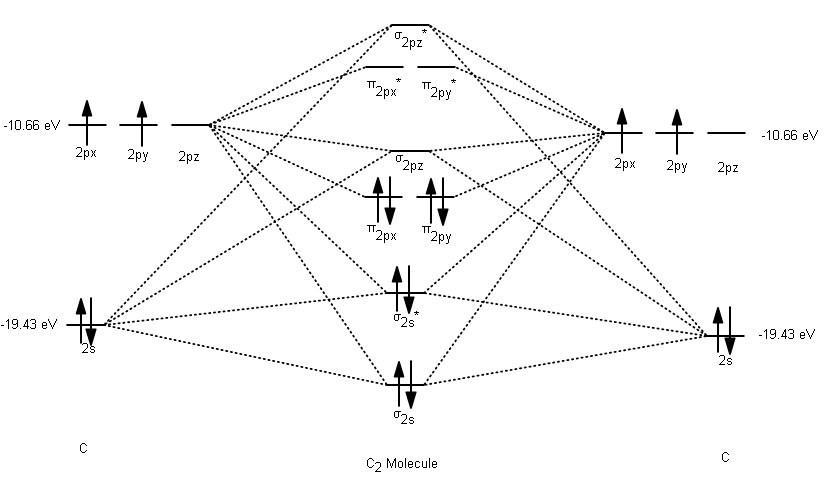
38 cs2 molecular orbital diagram
The CS2 molecular geometry is a diagram that illustrates the number of valence electrons and ... But carbon atom has s and p orbitals in the ground state.
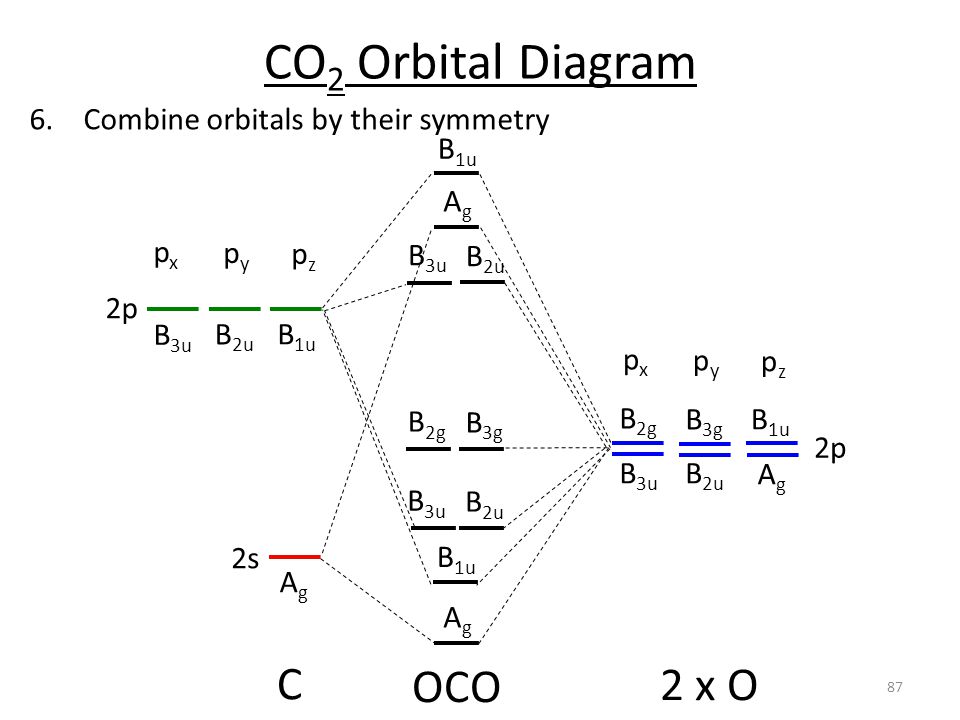
Solution for MO diagram for CS2, showing all of the possible orbitals for each atom or group. Show which interactions would be bonding, non-bonding, or…
all 6 substituents are the same Hybrid Orbitals. It gives distribution of orbital around the central atom in the molecule. There are 3 bonds to the Se which also has a lone pair (AX 3 E). The F-S-F bond angle in SF 2: The S is bound to two fluorines and has two lone pairs of electrons. 5°.

Cs2 molecular orbital diagram
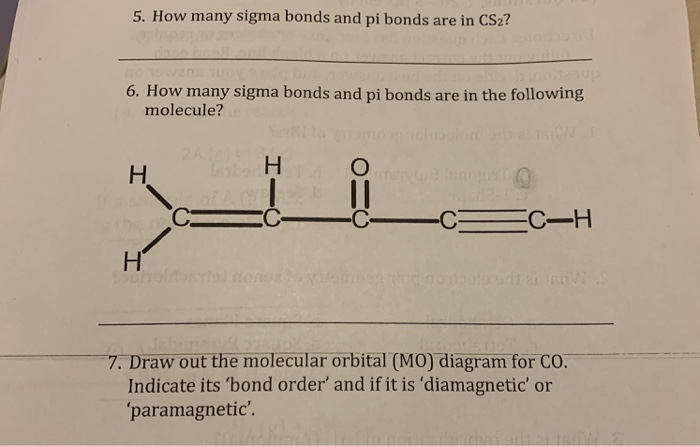
Apr 24, 2020 — (c) CS2. (d) AlCl3. (e) CH4. 19. Which of the following molecules has ... Using the appropriate molecular orbital diagram, what is the bond ...9 pages
Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF. Download. Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF
Question: Draw the MO diagram for CS2 . Showing the possible orbitals for each atom or group. Which would be bonding, non-bonding or antibonding?
Cs2 molecular orbital diagram.
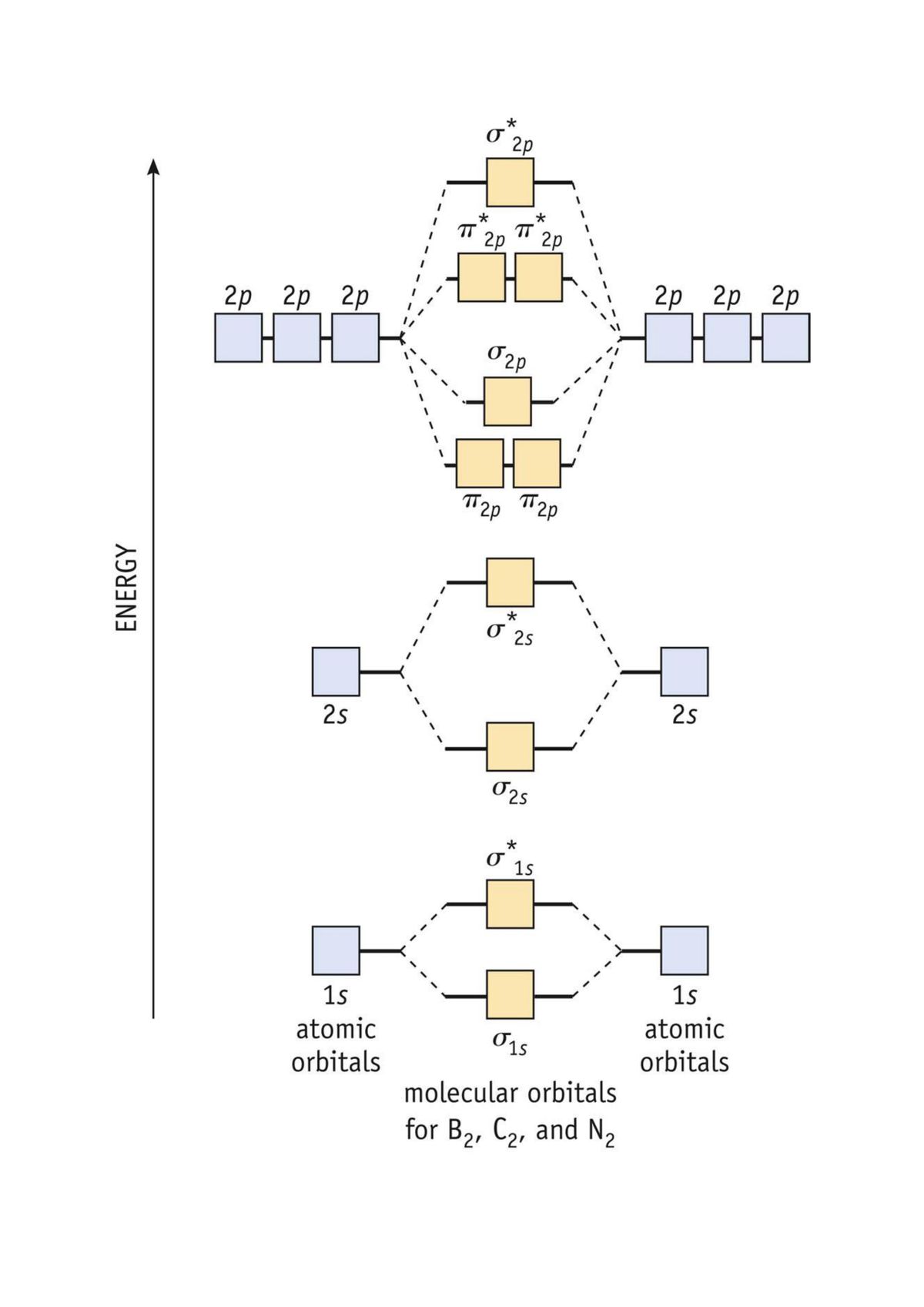
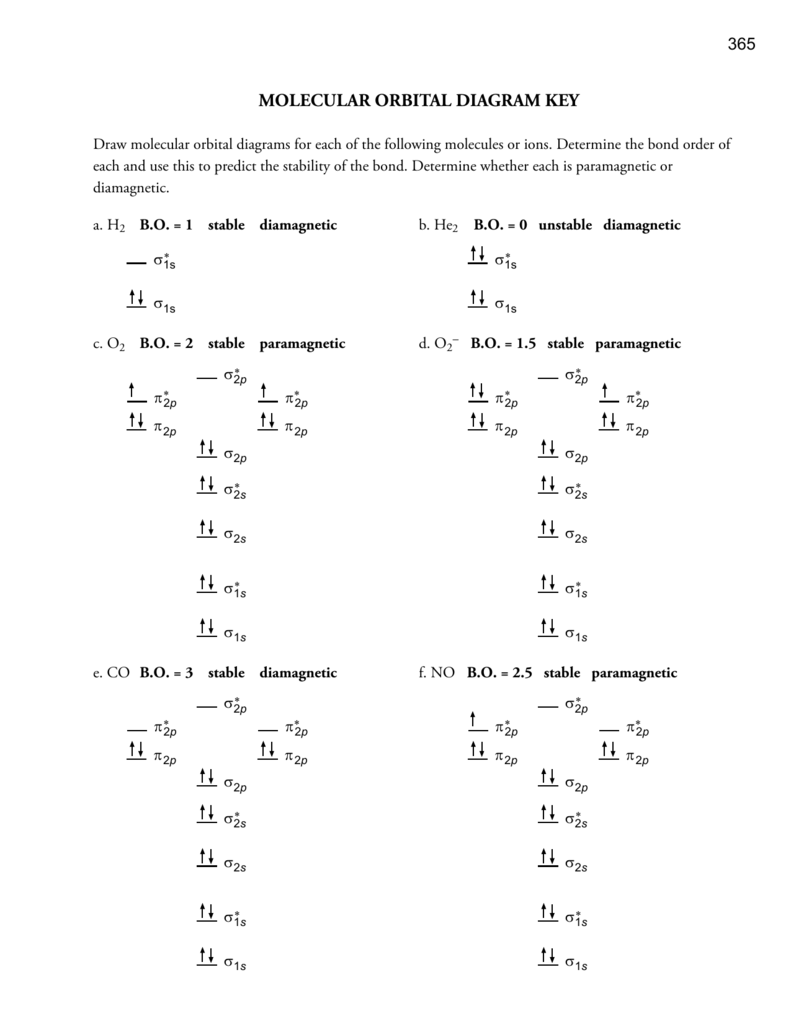
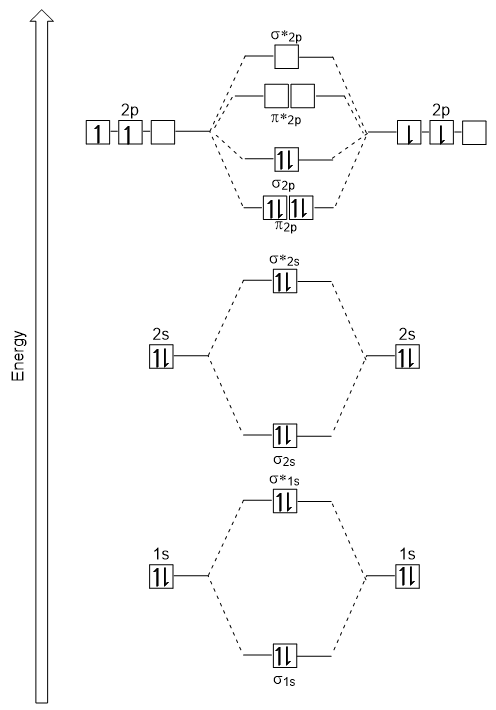
39. The energy of σ2pz molecular orbital is greater than π2px and π2py molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species : N 2, N 2+, N 2-, N 2 2+ Solution:
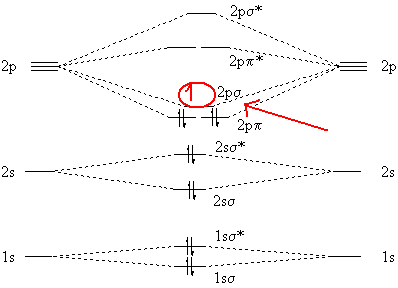
1984 · Cited by 78 — Interaction diagram for the molecular model (PH,)3NiCS2. The CS2 molecule is bent and bonded through the carbon atom to the metal. The molecular symmetry is ...10 pages
Provide the following information for the molecule CS2. a. electron geometry b. molecular geometry c. bond angles d. polarity e. central atom hybridization . ... Write orbital diagram (boxes with ...
Feb 15, 2021 — In CS2 molecule, two double bonds are formed consisting of eight valence electrons. Thus it takes up eight valence electrons out of 16 valence ...
B) An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy. C) It is possible for two electrons in the same atom to have identical values for all four quantum numbers. D) Two electrons in the same orbital can have the same ...
Draw the MO diagram for CS2, showing all of the possible orbitals for each atom or group. Show which interactions would be bonding, non-bonding, or anti-bonding ...4 answers · Top answer: Let's look at the bonding in the molecule. So for diet fluoride, So so far I fluoride is composed ...
Draw the molecular orbital diagram shown to determine which of the following is most stable. O22, C22- Choose the compound below that contains at least one polar covalent bond, but is nonpolar.
Carbon disulfide or CS2 is one of the very common molecules we come across ... Step 6: The final step of Lewis diagram formation is to verify whether all ...
Nov 20, 2021 · Key Points To Consider When drawing The SCl4 Molecular Geometry. A three-step approach for drawing the SCl4 molecular can be used. The first step is to sketch the molecular geometry of the SCl4 molecule, to calculate the lone pairs of the electron in the central sulfur atom; the second step is to calculate the SCl4 hybridization, and the third step is to give perfect notation for the SCl4 ...













0 Response to "38 cs2 molecular orbital diagram"
Post a Comment