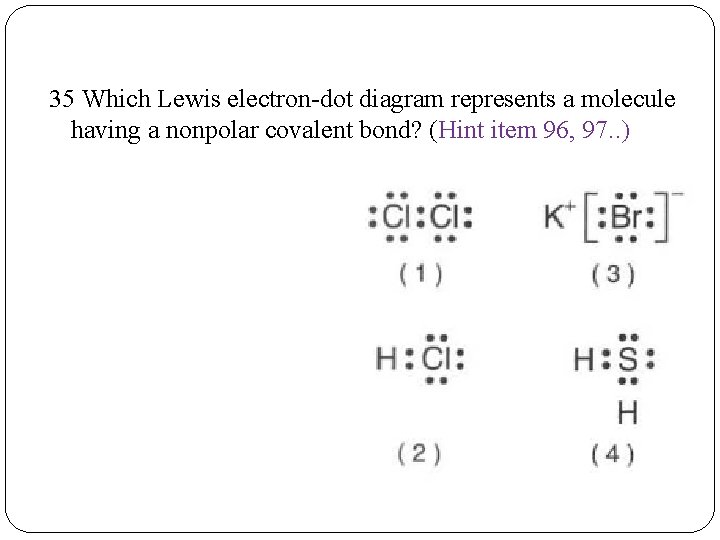
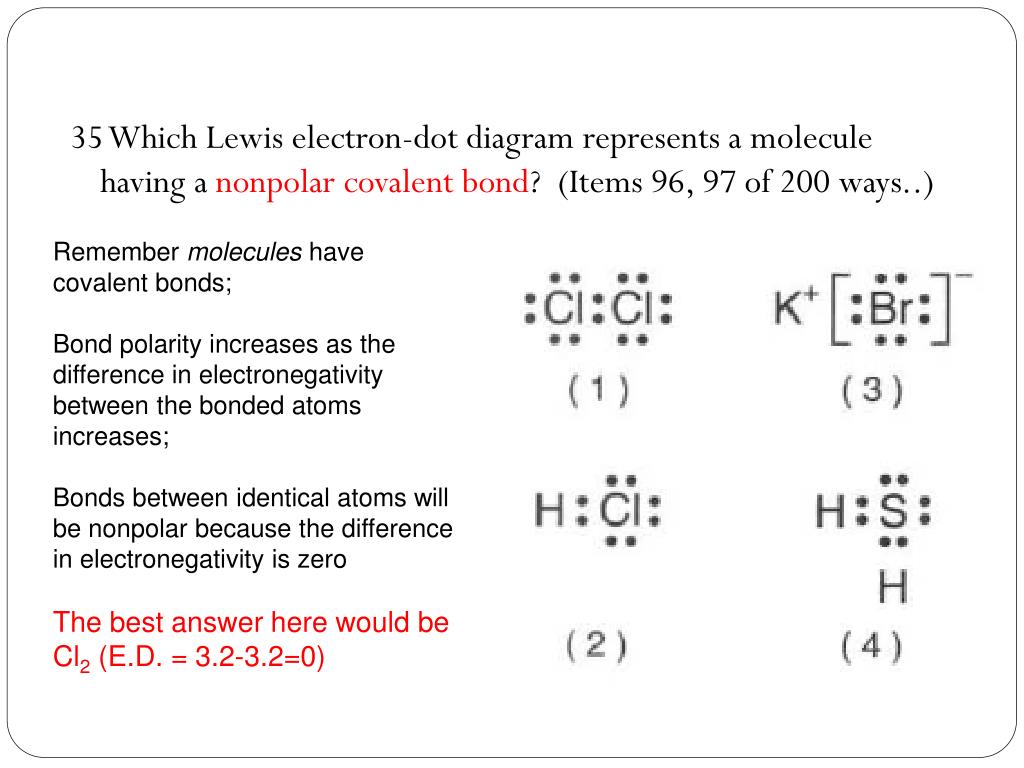
37 which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond?
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in a molecule. While it can be helpful initially to write the individual shared electrons, this approach quickly becomes awkward. A single line is used to represent one pair of shared electrons.
Some students prefer a more formal series of steps for drawing Lewis structures for a molecule, given the formula. Mueller inserted these three steps below, with images of two examples that follow (HNO and NaOCl). STEP 1: Draw the Lewis dot structure for each atom in the formula. (Draw a dot for each valence electron around each atomic symbol).
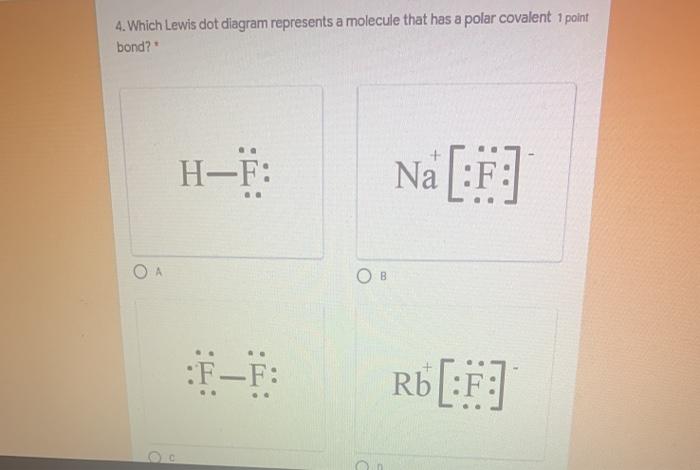
Jan 16, 2018 · 1 answerInterlocking circles are seen in the electron dot diagram that represents a molecule that has a polar covalent bond.

Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond?
Lewis dot structures or Lewis structures are the diagrams that help to understand the bonding of atoms along with the lone pairs present in the molecule. The valence electrons of atoms form bonds, and these bonds are represented by showing straight lines. Each bond takes up two valence electrons, and the excess electrons are showed as lone pairs.
7 In a nonpolar covalent bond, electrons are ... What is the correct Lewis electron-dot diagram ... 23 Which electron dot diagram represents a molecule.
A Lewis Structure is a very simple representation of the valence, or outermost, electrons in a molecule. It does not explain the geometry of the molecule, but it is a step forward in approaching the geography. But to find out if N2 is polar or nonpolar, the Lewis Structure can reveal the best electron makeup of the molecule.
Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond?.
Lewis structure is a theory that helps in understanding the structure of a given compound, based on the octet rule. According to the octet rule, a molecule should have eight electrons in its outer shell to become inert or stable. For this compound, there is one molecule of Carbon, two molecules of Hydrogen and two molecules of Chlorine. To know ...
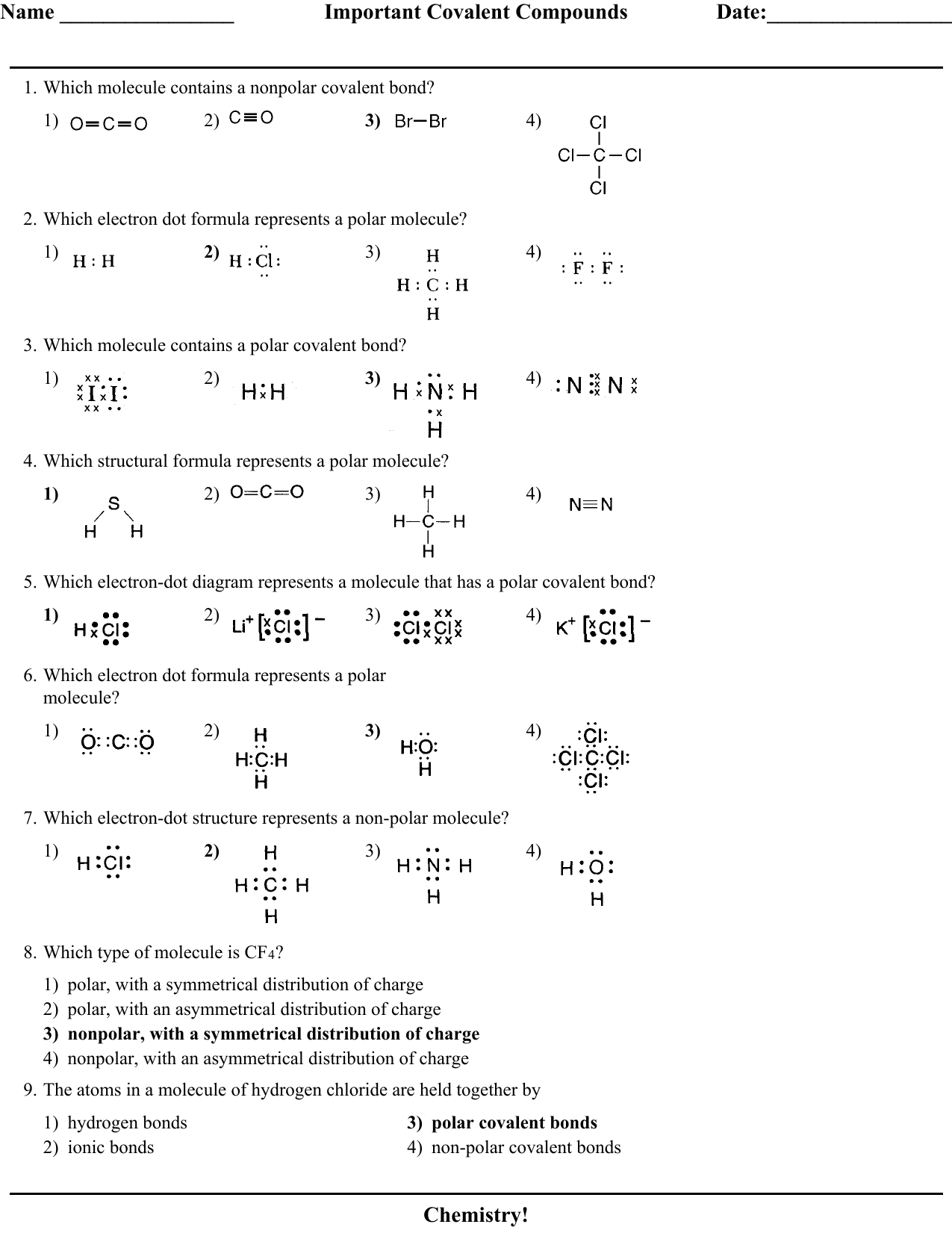
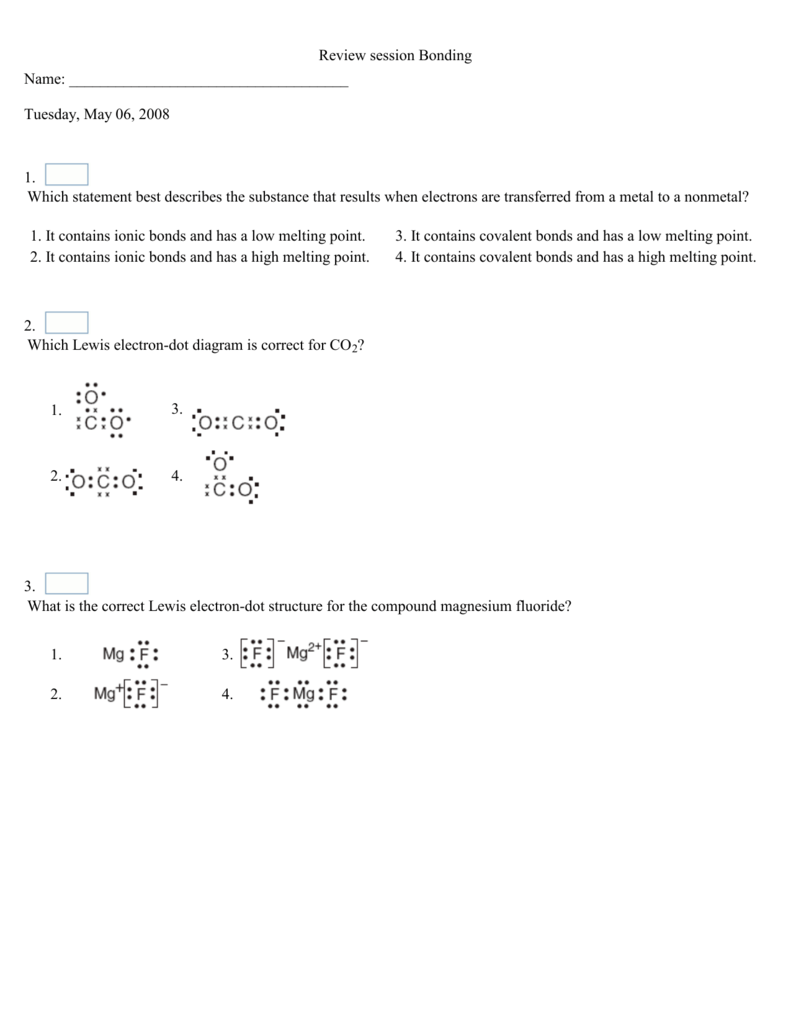
Which Lewis electron-dot diagram represents the bonding in potassium iodide ... Which formula represents a nonpolar molecule containing polar covalent bonds ...12 pages
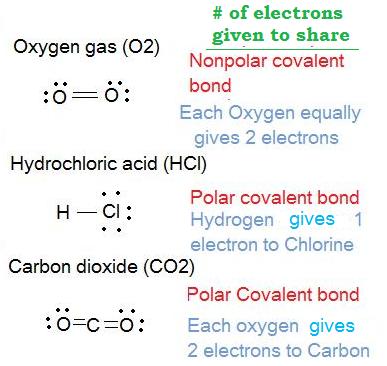
Nonpolar Covalent Bond When electrons are equally shared between the combining atoms, a nonpolar covalent bond is formed. This phenomenon happens when there is no difference in the electronegativities of the two combining atoms. That is, to say, identical pairs of atoms form a nonpolar covalent bond.
Lewis Structure is also known as an electron-dot structure since it uses dot notations to represent the valence shell electrons in the skeletal diagram. Here, as we can see, we have put all the 42 electrons surrounding the six atoms in ClF5. Since Chlorine is the central atom here, it will form bonds with all the five Fluorine atoms.
Instead of having polar bonds, the molecule is nonpolar. Lewis Structure and Geometrical Structure of CS2 Lewis structure of a molecule is also known as electron dot structure because it represents the number of valence electrons of the molecule that take participation in the bond formation.
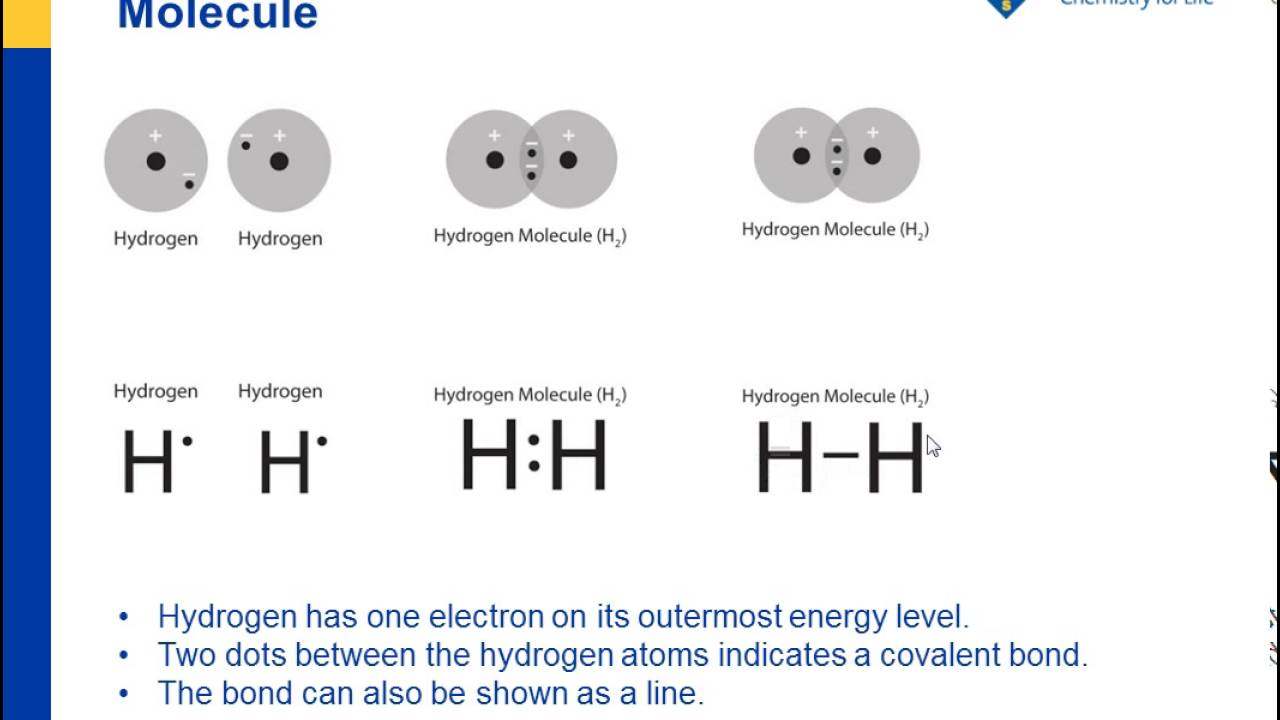
As a chemical bond forms between two hydrogen atoms ... Which electron-dot diagram represents H2? ... Which molecule will have a double covalent bond?7 pages
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. f o r m a l c h a r g e ( N) = 5 − ( 0 + 8 2) = 0 .
Lewis structure is also referred to as electron dot structure. Dots are represented to show the electrons, and lines are used to indicate the bonds between the atoms. Lewis structures are based on the octet rule, which says an atom must have eight valence electrons in its outer shell to attain a structure similar to the closest noble gas.
Take the case of NaCl (table salt). Chlorine contains 7 electrons in its outer shell and needs one to complete its octet. Sodium has 1 electron in its outer shell, which it donates to chlorine to form an ionic bond. Covalent Bond. A covalent bond occurs when electron pairs are shared between two atoms; it is also the strongest of the chemical ...
Lewis Structure of KrF2 Lewis dot structure is a pictorial representation of covalent bonding between the combining atoms. The valence electrons of each atom are represented in Lewis structure. In KrF2 molecule, there are a total of 22 valence electrons present (8 from krypton and 14 from both fluorine).
Which Lewis electron-dot diagram correctly represents a ... Which formula represents a molecular compound? 1) CO2(g) ... having a nonpolar covalent bond?6 pages
What is the molecular shape of cocl2? Be certain you include any lone pairs. Lewis Electron - Dot Structure Of The Nitrite Ion No2- Chemistry Classroom Chemistry Help Organic Chemistry Discover and download thousands of 3d models from games cultural heritage architecture design and more. Draw the lewis structure for cocl2 including lone pairs.
Which molecule or ion has a trigonal pyramidal molecular geometry! a. We only have one of those. H2CO lewis's structure is made up of one oxygen, one carbon, and two hydrogens. Lewis dot structure of H 2 CO. 05a Determine the formal charg on atoms in NH4+ 2:24. H2CO lewis's structure is made up of one oxygen, one carbon, and two hydrogens.
Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2. Using Lewis structures, we can represent this as follows: Two fluorine atoms can form a molecule of F 2 in the same fashion. Note that each atom must contribute one electron to the bond. Atoms can form more than one bond.
Which Lewis electron-dot diagram represents a molecule having a nonpolar covalent bond? Answers. montanolumpuy. 1. Compound that contains both ionic and covalent bond is . 2. Electronegativity difference is high in water. Explanation: For 1:
1. by asserting that electrons in a double bond can delocalize (spill over) onto adjacent single bonds to make a bond and a half. 2. by asserting that double bonds "flip" or resonate between two locations in the molecule. 3. where there is more than one choice of location for a double bond as deduced from Lewis dot structures.
Which electron-dot diagram represents a molecule that has a polar covalent bond? ... 2) N‡‡H bonds are nonpolar. 3) NH3 molecules have asymmetrical.4 pages
Lewis structure of a molecule is the structure that depicts the bonding between the atoms and lone pairs on the atoms involved in the molecule Lewis structure can be drawn for coordination compounds as well as covalent compounds. It is also known as an electron dot diagram.
Pcl3 geometry molecular nonpolar electron vsepr valence polar shell lewis structure pcl pair study short. Lewis Dot Diagram For Pcl3 Wiring Diagram. Lewis concept acid is the substance that accepts a lone pair of electrons since it has empty orbitals in the valence shell The phosphorus in PCl5 readily accepts electrons from other.
Molecular geometry is the 3-dimensional diagram showing how electrons have bonded to determine a concrete value for bond angle, bond type, and molecular structure, and other geometrical parameters.
Polar Bond Definition . A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed. This causes the molecule to have a slight electrical dipole moment where one end is slightly positive and the other is slightly negative. The charge of the electric dipoles is less than a full unit charge, so they are considered partial charges and denoted ...
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds.
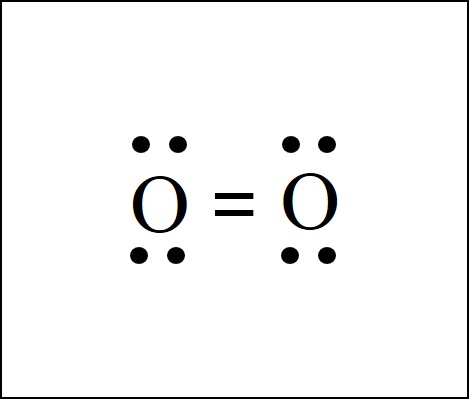
The O2 molecule forms a double covalent bond between two shared pairs of electron s. Lewis Dot Structure Ionic Bonds Worksheet Pdf. Lewis Dot Structure Practice Worksheet Awesome Chemistry 162 Exam Study 2 Guide In 2021 Chemistry Worksheet s Chemistry Education Organic Chemistry Study.
A Lewis dot structure is also called a Lewis structure a Lewis dot diagram an electron dot structure or a dot diagram. Lewis Dot Diagrams of Selected Elements. To draw the lewis dot structure of co2 we have to find out the valence electrons of carbon and oxygen firstwe express valence electrons as dots in lewis dot structure.
Given the electron dot diagram: ... a nonpolar covalent bond? 18. Draw the electron-dot (Lewis) structure of ... Which electron dot formula represents a.3 pages
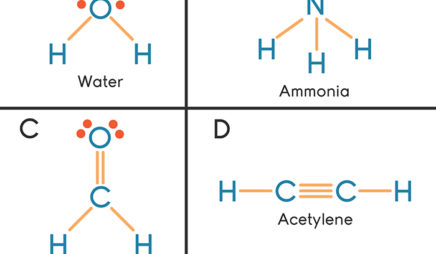
Covalent bonds are formed when atoms share electrons. Lewis electron dot diagrams can be drawn to illustrate covalent bond formation. Double bonds or triple bonds between atoms may be necessary to properly illustrate the bonding in some molecules. Contributions & Attributions
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds.
A Lewis dot structure is a diagram that shows the valence electrons in an element. In a Lewis dot structure, the nucleus of the element is represented by its symbol. The valence electrons are...
SF2 has a simple Lewis structure in which the Sulphur atom is in the centre forming single bonds with both the Fluorine atoms. There are two lone pairs of electrons on the Sulphur atom which makes the geometry of the molecule bent. The Sulphur atom has sp3 Hybridization, and the bond angle of F-S-F is 98 degrees.
Nov 17, 2016 · 2 answersWhen you draw the lewis dot structure can you draw a circle around both atoms that represent a stable inert gas structure. Explanation:.














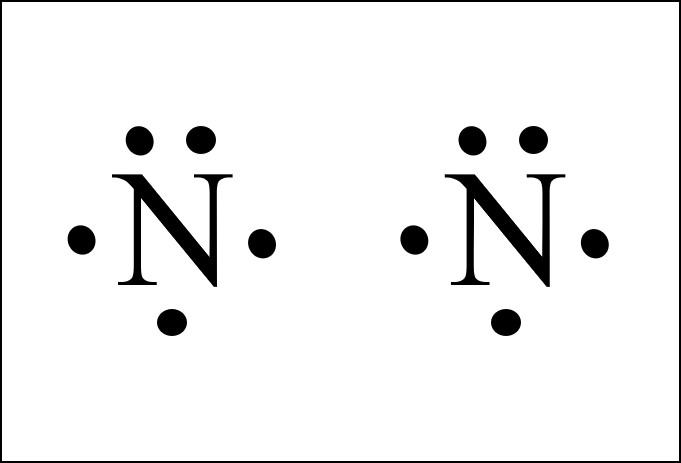



0 Response to "37 which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond?"
Post a Comment