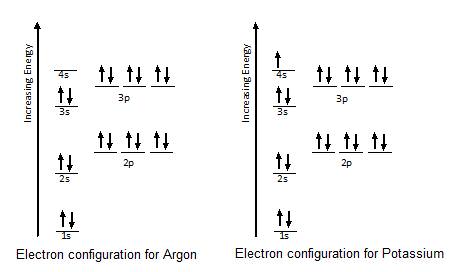
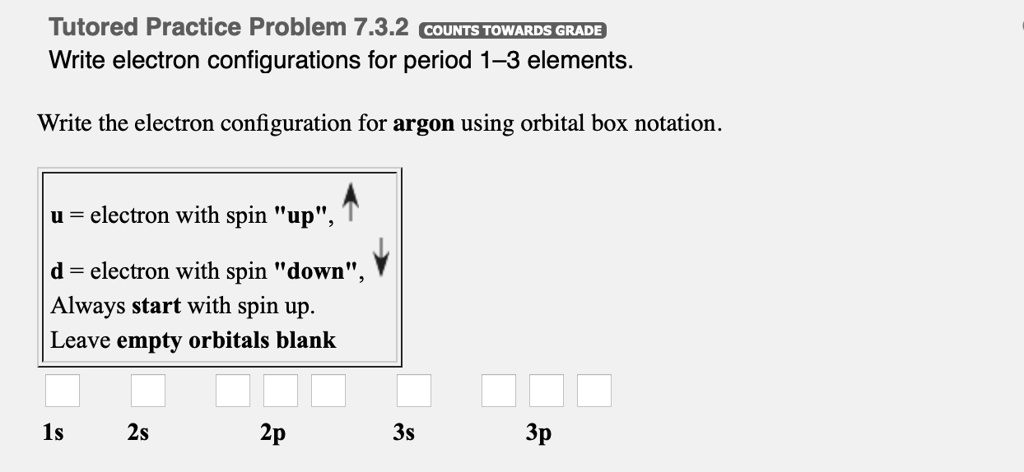
37 orbital diagram for argon
An orbital diagram helps to determine the electron configuration of an element. Electrons that occur together in an orbital are called an electron pair. The state in which all electrons have the lowest possible energy. Draw the mo diagram for molecular oxygen, o2. The orbital diagram is also one way of representing the electron configuration. Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams. In line with the Auf Bau Precept, every electron occupies the bottom power orbital. You soar up a bit bit in power and we get the 2s orbital that make it the 2p sublevel.
It is written out, as opposed to orbital diagrams which are depicted pictorially. Write the shorthand electron configuration for each of the following. Oganesson (element 118 is a good example to show the order of the orbitals. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas.
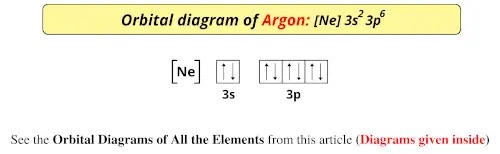
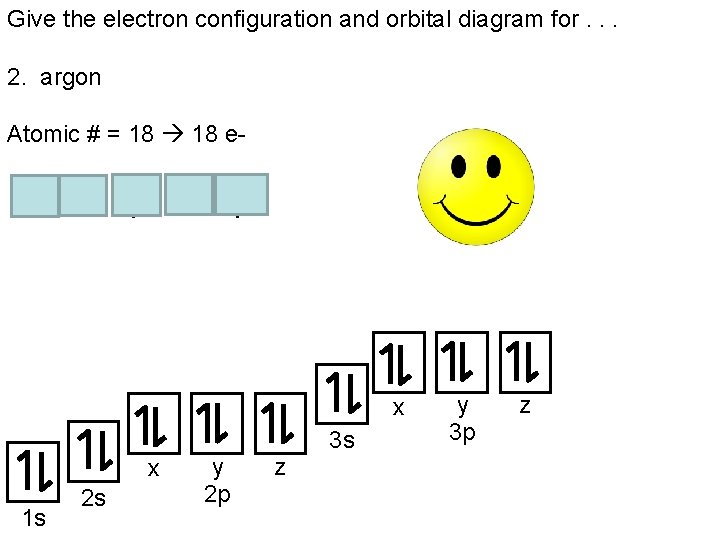
Orbital diagram for argon
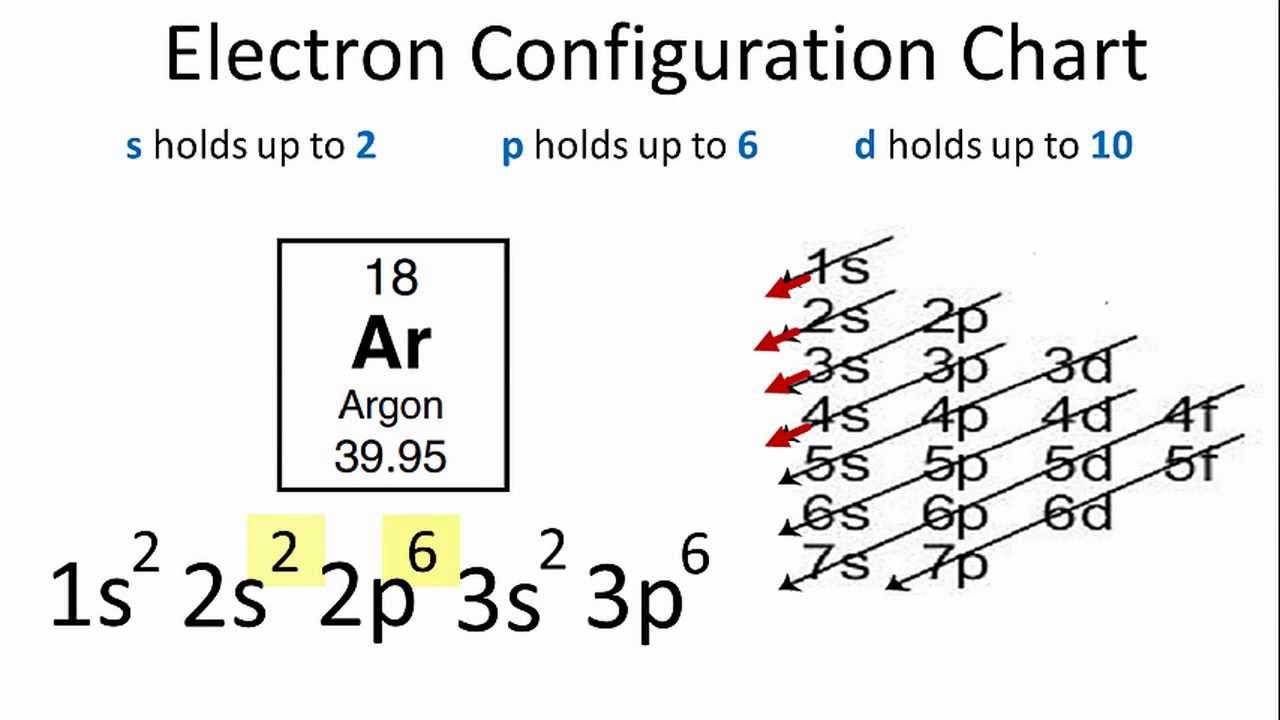
Argon (Ar) has an atomic mass of 18. Find out about its chemical and physical ... Electron Configuration, [Ne] 3s2 3p6. 1s2 2s2 2p6 3s2 3p6. Orbital Diagram. This row concludes with the noble gas argon, which has the electron configuration [Ne] 3s 2 3p 6, corresponding to a filled valence shell. Example 2.2.2. Draw an orbital diagram and use it to derive the electron configuration of phosphorus, Z = 15. What is its valence electron configuration? Given: ... Aug 15, 2020 · Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.
Orbital diagram for argon. The orbital ellipse itself precesses in space, in an irregular fashion, completing a full cycle every 112,000 years relative to the fixed stars. Apsidal precession occurs in the plane of the ecliptic and alters the orientation of the Earth's orbit relative to the ecliptic. This happens primarily as a result of interactions with Jupiter and Saturn. Earlier I was listening to [a podcast about neutrinos](http://www.bbc.co.uk/programmes/b0106tjc), and at one point someone mentions looking for argon atoms amongst [...a different kind, let's say helium for simplicity's sake] atoms as proof of something. I remember those diagrams in my text books, with the little nucleus and the orbiting electrons, but obviously that's not what you see under a scanning idontknowtheterm microscope or whatever the technology is. What do you see when you look at an... Noble Gas Crystal Structure. Argon cost 280 or 480 argon comes from the Greek word argos meaning lazy or inactive. Bohr Model Diagram Cards Bohr Model Super Teacher Worksheets Homeschool Kindergarten Figure 2 contrast the Bohr diagrams for lithium fluorine and aluminum atoms. Bohr diagram for argon. Last class we determined that the Bohr Model […] Below is the electronic diagram of the Argon atom. Orbital diagram of the Argon atom. Distribution of electrons over energy levels in the Ar atom
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿ 3d: ... With argon-nitrogen gas mixture, the Ti interlayer becomes stronger and thus can provide increased support to the TiB2 coating, leading to enhanced adhesion strength. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...18 Nov 2013 · Uploaded by Wayne Breslyn Mar 14, 2019 · Molybdenum disulfide is naturally inert for alkaline hydrogen evolution catalysis, due to its unfavorable water adsorption and dissociation feature originated from the unsuitable orbital orientation. That way this d orbital or d sublevel be completely filled which is very stable versus the s orbital will be halfway filled. So, again, I'm going to make this argon just make it for myself 4s1, 3d10 and again you might see it as argon 3d10, 4s1 same exact thing.
A molecular orbital is a region of space in a covalent species where electrons are likely to be found. The combination of two atomic orbitals always forms two molecular orbitals; the bonding molecular orbital, which is _____ in energy, and the antibonding molecular orbital, which is _____ in energy, than the original atomic orbitals. What is orbital diagram method? An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. 14+ Orbital Diagram For Sulfur. 2 electrons occupy the completed first energy level (k shell), 8 electrons. Well, we use the #aufbau principle#, and for sulfur, #z=16#. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18. If you dont have a chart you can still find the electron configuration. However notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for Argon a noble gas. This means that there are two electrons in the 1s orbital and one electron in the higher energy 2s orbitalElectron Configuration for Calcium CaWhat is the orbital notation of calcium.
It is written out, as opposed to orbital diagrams which are depicted pictorially. Write the shorthand electron configuration for each of the following. Oganesson (element 118 is a good example to show the order of the orbitals. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas.
Below are sample joints produced by orbital welding using of argon back-up gas with different level of oxygen. To avoid oxidation, we recommend an oxygen monitoring device to monitor the oxygen level in the backing gas. It is best to use an O2 monitoring device which is capable to be integrated and inter-locked to an orbital welding system.
There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagram s that you can save for free. Orbital diagram s are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagram s. According to the Auf Bau Principle, each electron occupies the lowest energy orbital.
Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of ...
24 Jan 2021 — 1s only hold two electrons and the next 2 electrons for Argon goes in the 2s orbital. The next six electrons go to 2p orbital. The p orbital ...
Here is the orbital notation for 25Mn. 25Mn 1s2 2s2 2p6 3s2 3p6 3d5 4s2. For the box diagram, note that all of the orbitals are FULL except 3d5 so place two electrons in each box for all except 3d5. For the 3d box place 1 electron in each of the 5 boxes. 👍.
Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration. ... How many must be thinking that what exactly is it, so to make it easier, the atomic number of the element Argon is 18 and its symbol is written as Ar? Therefore in the electronic configuration, it is very big in length to write its full electronic configuration, so the ...
The term "orbital" was coined by Robert Mulliken in 1932 as an abbreviation for one-electron orbital wave function. However, the idea that electrons might revolve around a compact nucleus with definite angular momentum was convincingly argued at least 19 years earlier by Niels Bohr, and the Japanese physicist Hantaro Nagaoka published an orbit-based hypothesis for electronic behavior as early ...
Argon Orbital Diagram. Electron Configuration exceptions. Occurs in certain transition metals due to the added stability associated with half-filled and filled subshells. Copper electron configuration [Ar] 4s1 3d10. Silver electron configuration
Answer (1 of 2): In strong field delta0 will be more. Co =3d74s2 Co +3= 3d6 CN- is considered as strong ligand, hence all 6 electrons will be filled in t2g orbitals. All paired. Diamagnetic, inner orbital complex, d2sp3. Now in case of Br- is considered as weak ligand hence all 6 electrons wi...
From the orbital diagram, ... This row concludes with the noble gas argon, which has the electron configuration [Ne]3s 2 3p 6, corresponding to a filled valence shell. Example \(\PageIndex{1}\): Electronic Configuration of Phosphorus.
Nov 07, 2013 · According to the aufbau diagram the configuration should be 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 1 and indeed it is. But conventional wisdom claims that the final electron to enter the atom of scandium is a 3d electron, when experiments indicate that the 3d orbital is filled before the 4s orbital. Why the mistake occurs
Argon purging is used to prevent contamination. What Are the Different Types of Orbital Welding? There are three main types of orbital welding. Fusion Orbital Welding. This process is much like the TIG application of orbital welding except that there is no filler metal added to the pool. Instead, the two joint members simply fuse together.
Original prompt: [[WP] Bryan Wetherspoon, 26, an interstellar pilot, has just been made redundant owing to increased automation. Vengeful, he is now enroute to Persephone in 40 Eridani B to blow up Jupiter Command, the central Hive Mind of all AI systems across the human interstellar community.](https://www.reddit.com/r/WritingPrompts/comments/oxo78o/wp_bryan_wetherspoon_26_an_interstellar_pilot_has/) The talking head continued screaming, broadcasting his fury across *NeuroNet*. "Even...eve...
The diagram below represents the orbital representation diagram used in earlier chapters. The orbital representation diagram has a few different variations but all are used to draw electron configurations. Most show the orbitals in groups, as lines, boxes, or circles with each orbital having its own line (or circle) within each sublevel.
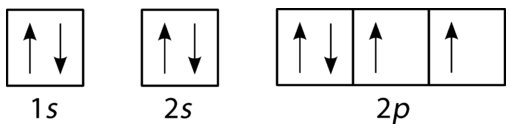
Boron (atomic number 5) has five electrons. Four electrons fill both the 1s and 2s orbitals. The fifth electron is added to a 2p orbital, the sublevel next higher in energy (Figure 5.9). The electron configuration of boron is: B: 1s 2 2s 2 2p 1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18.
The following is an orbital diagram for a nitrogen atom. Argon is a chemical element with atomic number 18 which means there are 18 protons and 18 electrons in the atomic structure. After putting 2 arrows in the first box called the 1s orbital, there is still another arrow to draw. It doesn't matter what order they are drawn, since they are ...
Electron Configuration Chart of All Elements (Full Chart) June 10, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
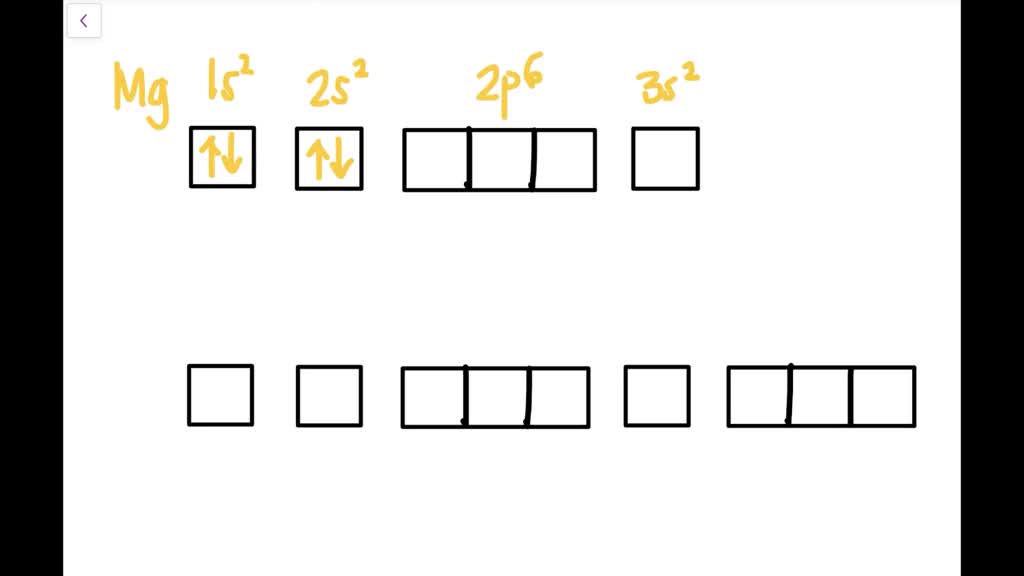
So scandium has the same configuration as argon, except with electrons in two extra orbitals. The shorthand form is therefore: [Ar] 4s 2 3d 1. Because the configuration of argon is: [Ar] = 1s 2 2s 2 2p 6 3s 2 3p 6. You can use this with any elements apart from hydrogen and helium. Orbital Diagrams
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
They are helium neon argon krypton xenon and radon. In writing the electron configuration for Potassium the first two electrons will go in the 1s orbital. In order to write the Potassium electron configuration we first need to know the number of electrons for the K atom there are 19 electrons.
What did you need to answer this question— an orbital diagram or an electron configuration? There are two unpaired electrons. The orbital diagram was necessary (below). 11. Write the electron configuration for zirconium (atomic # = 40). 1s 22s 2p63s 23p64s 3d104p65s 4d 12. Write the configuration for argon (atomic # = 18). 1s 22s 2p 63s23p 13.
Another way to represent an electron configuration is through an orbital diagram. In an orbital diagram, orbitals are represented as boxes and electrons are represented by arrows (↑ or ↓), with two electrons occupying each orbital/box. Orbitals are labeled according to their principle energy levels and sublevels (1s, 2p, etc..). Helium ...
Argon is a chemical factor with the image Ar and atomic quantity 18. It's in group 18 of the periodic desk and is a noble fuel. ... Orbital diagrams are pictorial descriptions of the electrons in an atom. Three guidelines are helpful in forming orbital diagrams.
Aug 15, 2020 · Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.
This row concludes with the noble gas argon, which has the electron configuration [Ne] 3s 2 3p 6, corresponding to a filled valence shell. Example 2.2.2. Draw an orbital diagram and use it to derive the electron configuration of phosphorus, Z = 15. What is its valence electron configuration? Given: ...
Argon (Ar) has an atomic mass of 18. Find out about its chemical and physical ... Electron Configuration, [Ne] 3s2 3p6. 1s2 2s2 2p6 3s2 3p6. Orbital Diagram.

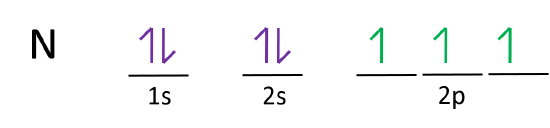














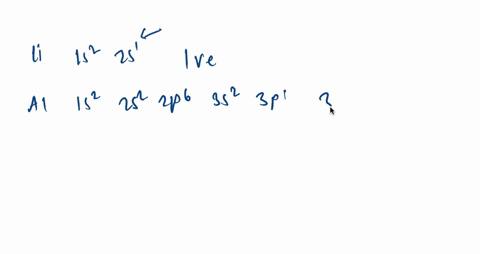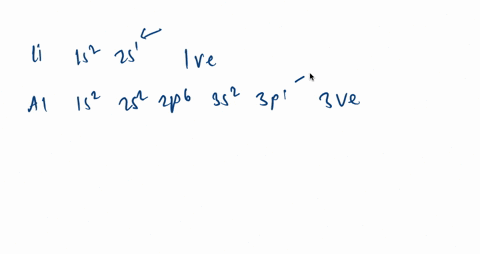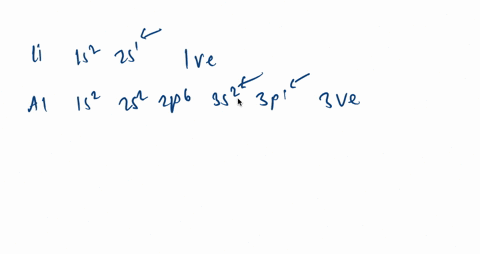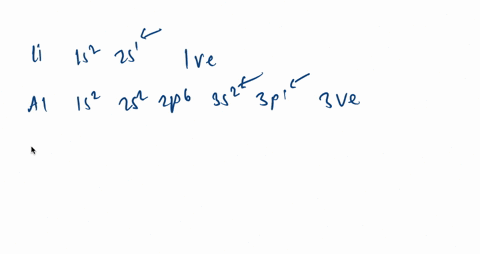







0 Response to "37 orbital diagram for argon"
Post a Comment