37 electron dot diagram for barium
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is ...
Answer: Electron dot structure is the valence electrons are represented by dots placed around the chemical symbol. Electrons are placed up to two on each side of the elemental symbol for a maximum of eightWhich is the number of electrons in a filled s and p shell. Electron dot structure of bariu...
Electron dot diagram for barium
Barium is a naturally occurring alkaline metalloid element with atomic symbol Ba, atomic number 56, and atomic weight 137 that is only found in combination with other elements, typically barite (barium sulfate) and witherite (barium carbonate), or chemicals. Barium is used in many industrial processes, as well as in diagnostic testing, fireworks, and pesticides.
This element exists in the solid state at room temperature and at normal atmospheric pressure and is found in emerald gemstones. It is known to be one of the following elements: carbon, germanium, sulfur, cesium, beryllium, or argon. Identify the element based on the electron-dot structure. a) Si 1s²2s²2p⁶3s²3p².
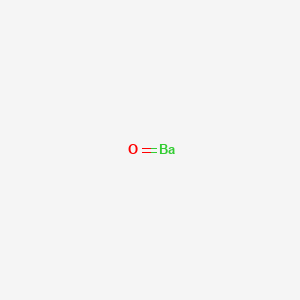
A step-by-step explanation of how to draw the BaO Lewis Dot Structure.For BaO we have an ionic compound and we need to take that into account when we draw th...
Electron dot diagram for barium.
Barium stimulates striated, cardiac, and smooth muscle, regardless of innervation. It is antagonistic to all muscle depressants, no matter whether they act primarily on nerve or muscle. Initial stimulation of contraction leads to vasoconstriction through direct action on arterial muscle, peristalsis through action on smooth muscle, tremors and cramps through action on the skeletal muscles, and ...
Barium iodide (BaI2) | BaI2 | CID 83684 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities ...
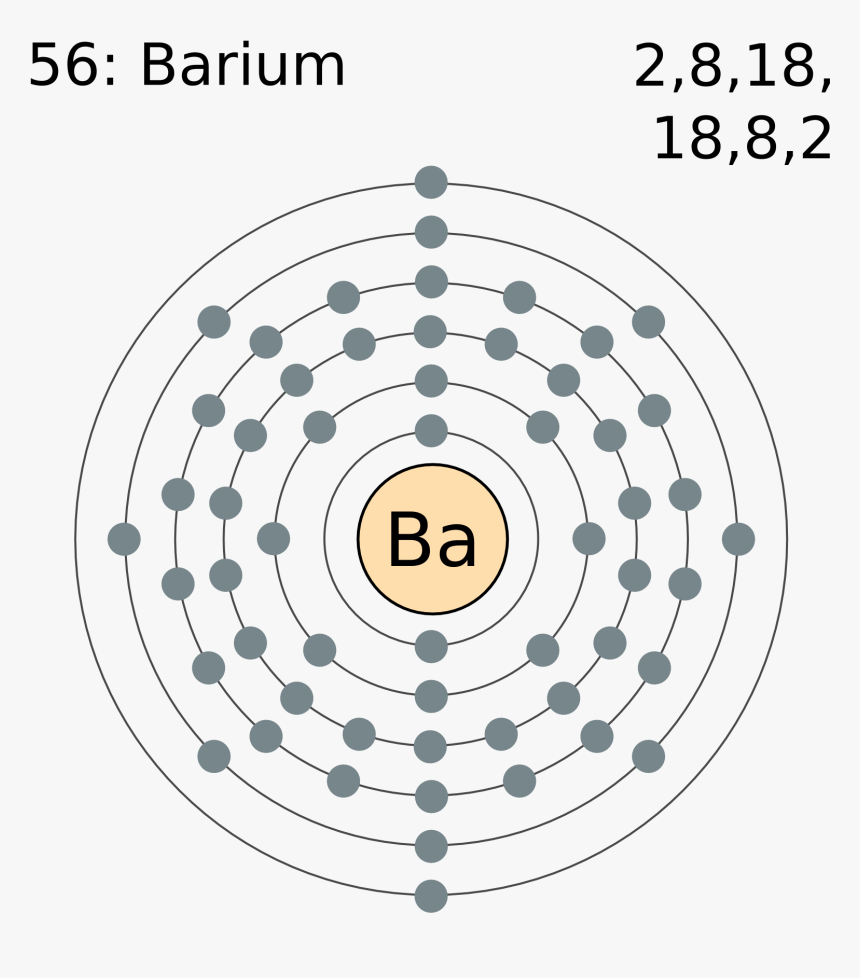
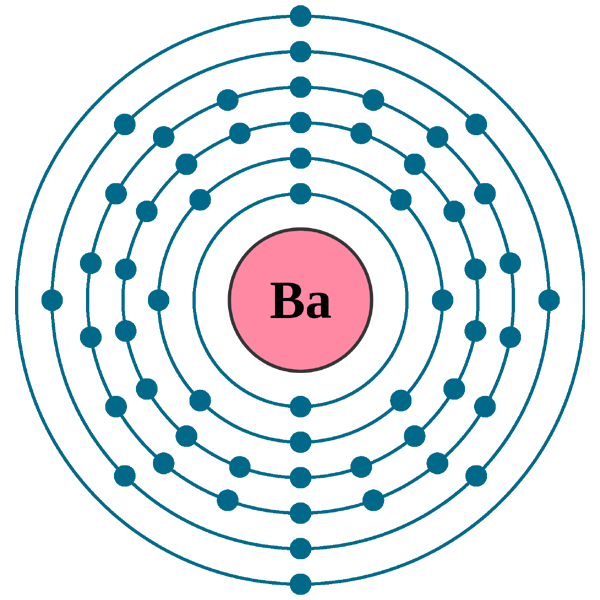
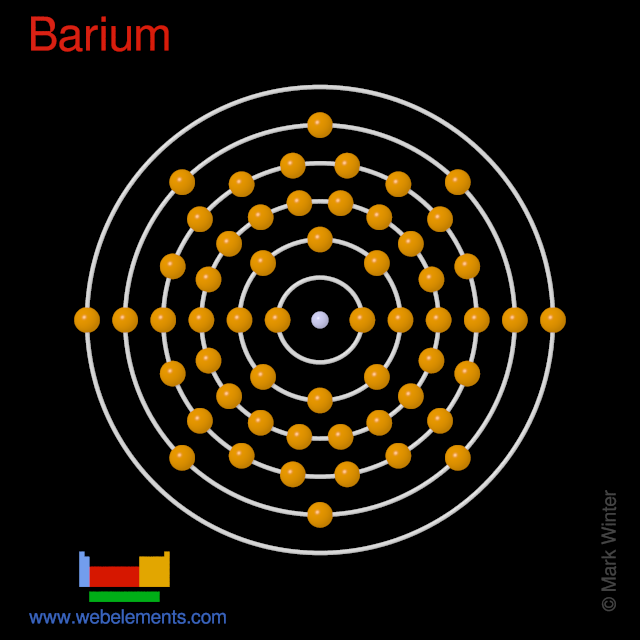
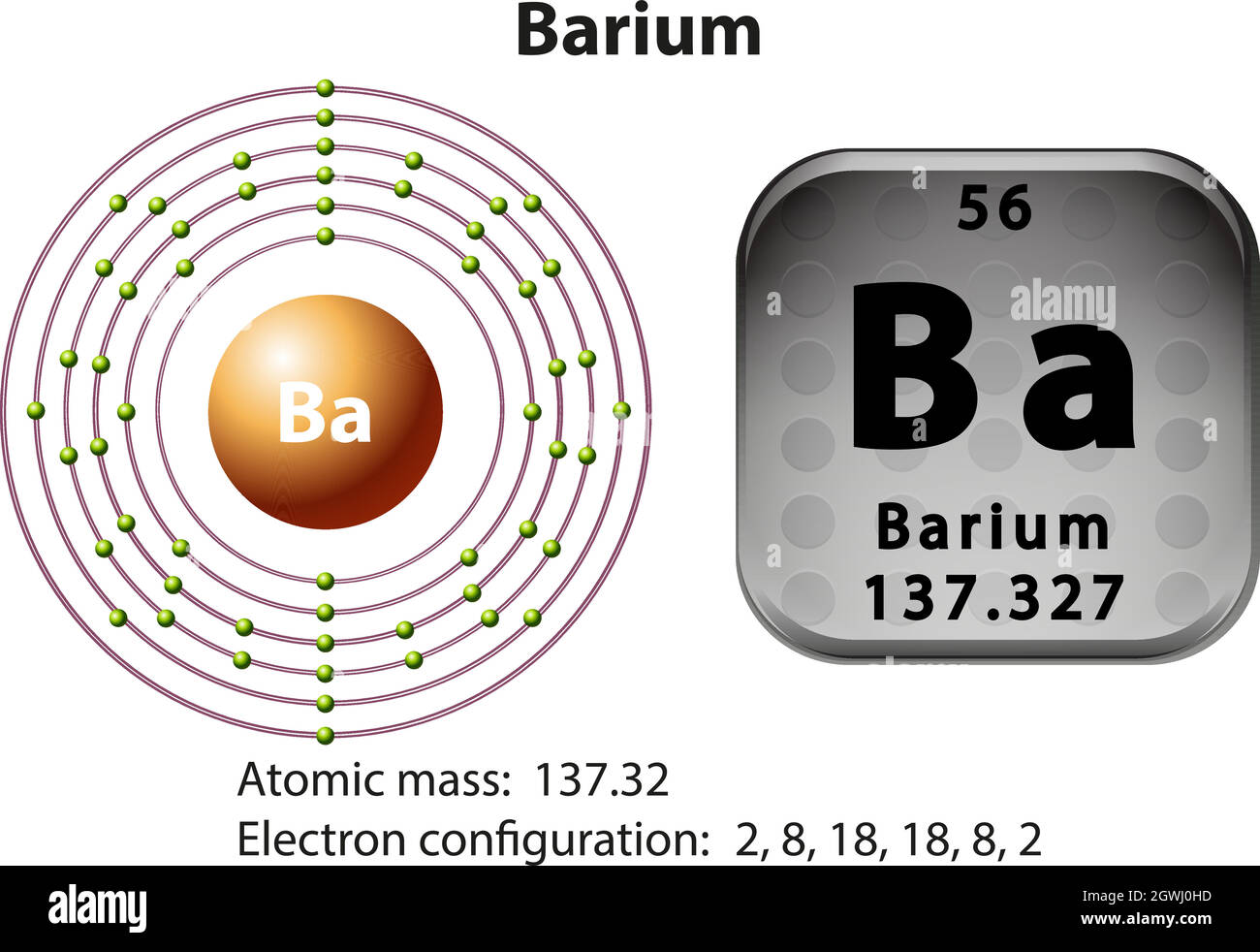
What is the second quantum number of the 3p1 electron in aluminum? 1. What are the 4 quantum numbers for barium? Barium Atomic and Orbital Properties Barium atoms have 56 electrons and the electronic shell structure is [2, 8, 18, 18, 8, 2] with Atomic Term Symbol (Quantum Numbers) 1S0.
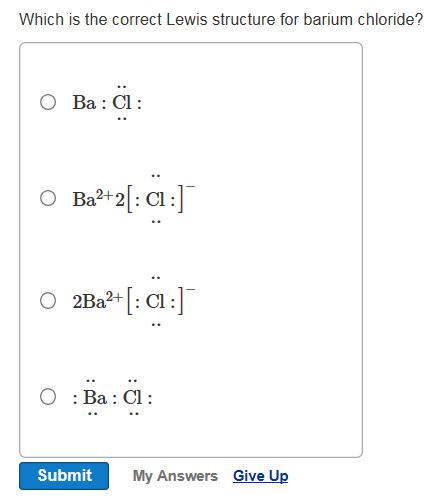
The Lewis structure for a barium Ba ion looks like this Ba2 what best describes what happens when a barium atom forms an ion? the barium atom loses two electrons.
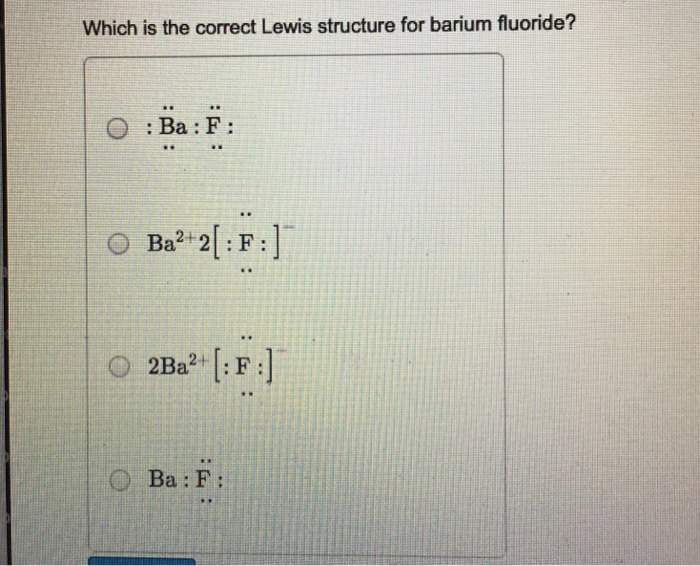
I searched for $\ce{BaF2}$ Lewis structure, and found out that the Barium atom loses its 2 valence electrons to form $\ce{F- Ba^2+F-}$. Before seeing it I thought each of $\ce{Ba}$'s valence electr...
XeO4 Lewis Structure, Geometry, Hybridization, and Polarity. XeO4 or Xenon Tetraoxide is a chemical compound made up of Xenon and Oxygen. It is prepared by treatment of barium perxenate with anhydrous sulphuric acid. It has a molar mass of 195.29 g/mol. It is exceptional for being a stable compound of a noble gas comprising of Xenon in its ...
Answer: Only two dots on the Be, since there are only two valence electrons. Your Cl will each have seven dots around it, with one of those seven alongside one of the dots on the Be atom to make an octet. You have two Cl atoms on either side of the Be.
electrons and draw the Lewis dot structure. 1. Barium 6. Carbon. 2. Tin 7. Krypton. 3. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) are . Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their. Column 1A 1 valence electron The first symbol in the column is H . electrons and draw the Lewis ...
Lewis theory predicts that the formula for a compound between barium and sulfur is: BaS. Lewis theory predicts that the formula for a compound between potassium and sulfur is: K2S. ... The total number of electrons to be counted for the Lewis structure of the PO43- polyatomic ion is. 32. The central atom in the chlorate anion, ClO3- is ...
BARIUM OXIDE reacts as a strong base. Combines exothermically with all categories of acids. Reacts with carbon dioxide to form barium carbonate [Merck 11th ed. 1989]. Ignites hydroxylamine on contact [Mellor 8:291 1946-47]. Mixtures with mercurous or nickel oxide react vigorously with hydrogen sulfide in air. Explosions may result [Mellor 10 ...
The lewis structure is F:Ba:F just draw the remaining electrons around the fluorine atoms (there is a total of 16 electrons in this compound). It is also non-polar (fluorine is most electronegative element in the periodic table and barium is not very electronegative but due to symmetry, the molecule is not polar).
Orbital Diagram For Barium. The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium. Barium is an alkaline earth metal. This means that it is a group 2 element. Barium's atomic number is 56; this means that it has 56 protons in its nucleus and also.
Uses of Barium: Used in sparkplugs, vacuum tubes, fireworks, fluorescent lamps. Insoluble barium sulfate is used for body imaging. Additional Notes: Must be stored under kerosene to remain pure. Soluble barium salts are highly toxic. Barium Menu. Barium Page One. Overview of Barium; Barium's Name in Other Languages; Atomic Structure of Barium
Barium chloride is an inorganic compound with the formula Ba Cl 2. It is one of the most common water-soluble salts of barium. Like most other barium salts, it is white, toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting first to the dihydrate BaCl 2 (H 2 O) 2.
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
Barium. Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. cesium ← barium → lanthanum.
Electron dot formula shows the number of valence electrons for that element with the help of dots. The valence electrons are those electrons that occupy the highest energy level. We can obtain it by using the periodic table. For example, the elements in group IA of the chemical periodic table have 1 valence electron.
A step-by-step explanation of how to draw the BaCl2 Lewis Dot Structure.For BaCl2 we have an ionic compound and we need to take that into account when we dra...
Electron dot diagram for barium. Therefore the lewis structure of the element has 7 dots. I show you where barium is on the periodic table and how to determine how many valence electrons barium has. Males 25 to 30 g and late gestation females 40 g were sacrificed after intravenous injection of 63 ug bariumkg 0063 mg bariumkg containing 133bacl2.
What is the electron dot diagram for barium? I cannot draw the 'dot diagram' in Answers. However, there are six electron shells. The number of electrons in each shell, starting from nearest to the ...
What is the electron dot diagram for barium? I cannot draw the 'dot diagram' in Answers. However, there are six electron shells. The number of electrons in each shell, starting from nearest to the...
Electron dot diagram for barium. The lewis structure is fbaf just draw the remaining electrons around the fluorine atoms there is a total of 16 electrons in this compound. The calcified tissues cartilage kidney and melanin containing tissues of the male and female pigmented animals had the highest 133.
Draw the Lewis structure of IF2O2¯ (with minimized formal charges) and then determine the ideal bonding angle(s) of the central atom. check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here. ... Aqueous barium nitrate i.e Ba(NO3)2 ...
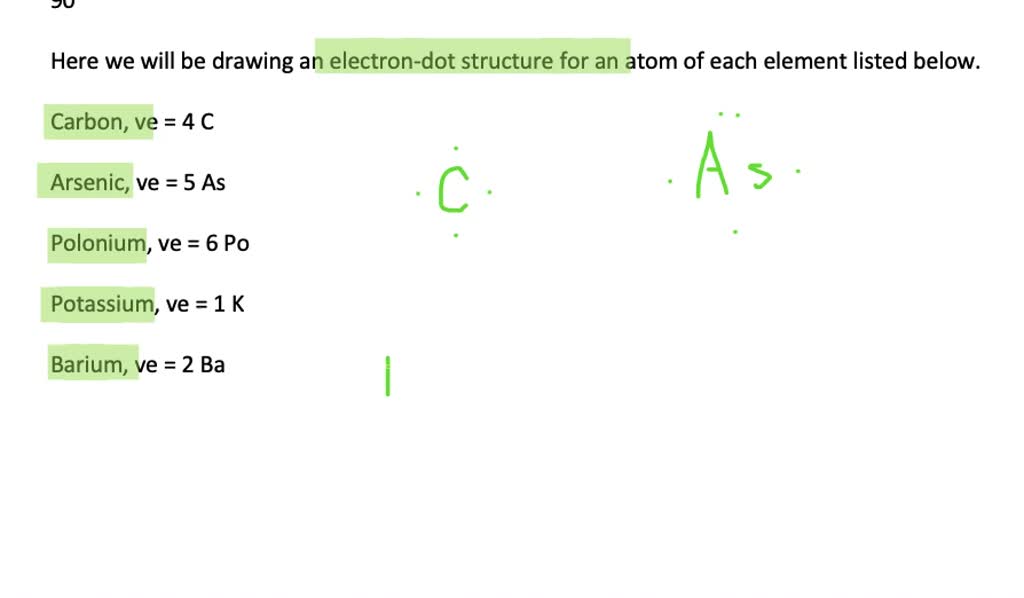
Draw an electron dot structure for an atom of each element a carbon b arsenic c polonium d potassium



/Lewis-dot-58f78f405f9b581d5938e617.jpg)







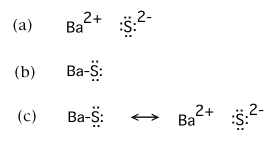


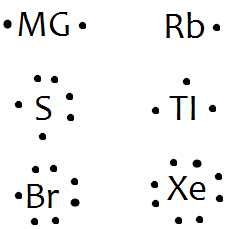
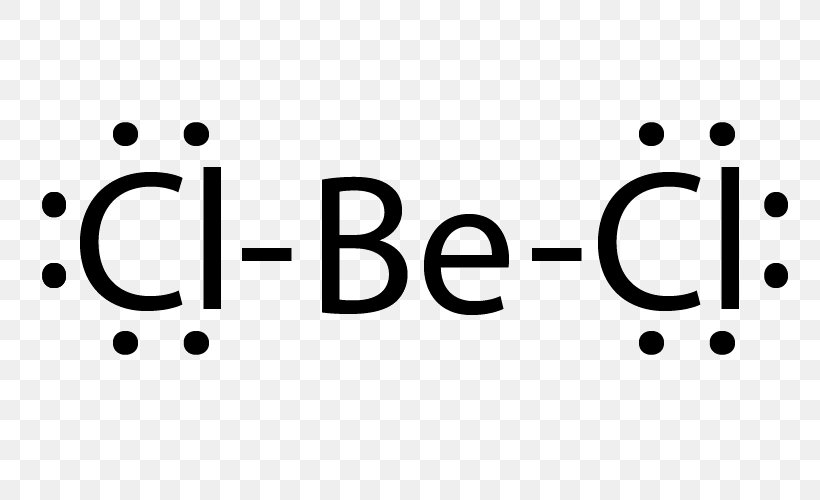







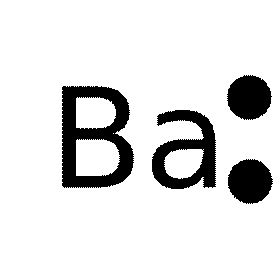
0 Response to "37 electron dot diagram for barium"
Post a Comment