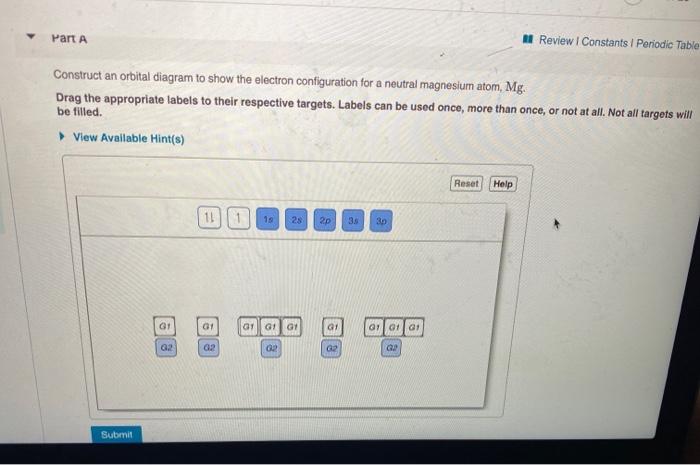
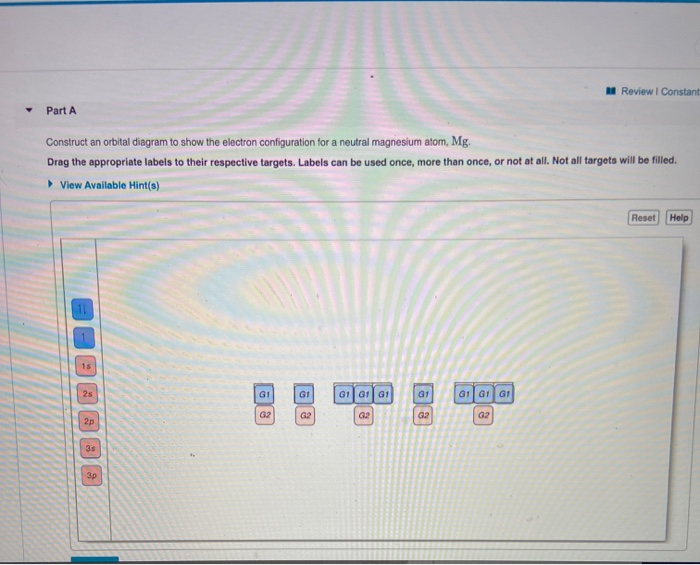
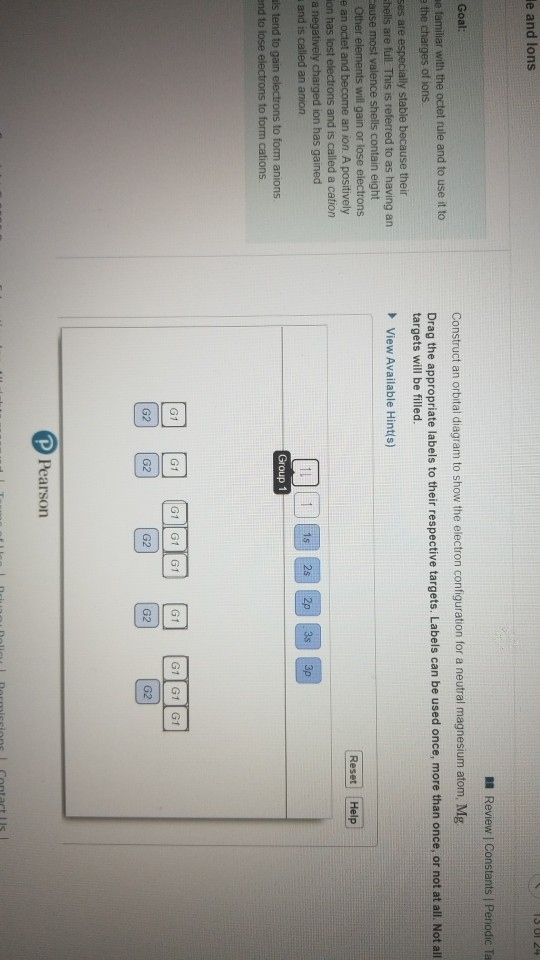
37 construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg.
Transcribed image text: Part A Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Use the buttons at the top ... Mg have 12 electrons in the neutral atom so the electronic configuration of the Mg is {eq}1s^2 2s^2 2p^6 3s^2 {/eq} Orbital diagram is as follows...
Transcribed image text: Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Use the buttons at the top of the ...
Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg.
FREE Expert Solution. We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg. Recall that for a neutral element, Atomic number = # of protons = # of electrons . The atomic number of Mg is 12 and since it’s a neutral element, this means Mg has 12 electrons. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the Magnesium atom. In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Use the buttons at the top of the tool to add sublevels.
Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg.. Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. 1s2, 2s2, 2p6, 3s2 To form a stable ion, will magnesium gain or lose electrons? Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, MgMg.Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled. Transcribed image text: Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Drag the appropriate labels to ... Transcribed image text: Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg Use the buttons at the top of the ...
May 17, 2015 · Short hand electron configuration for mg magnesium ar 4s2 3d10 4p2. Construct an orbital diagram to show the electron configuration for a neutral magnesium atom mg. Ionic Compounds Manoa Hawaii Edu Exploringourfluidearth How can we draw the orbital diagram of a magnesium atom and a magnesium ion. Answer to Construct an orbital diagram to show the electron configuration for a neutral magnesium atom,Mg Mg. Drag the appropriate labels to their ...1 answer · Top answer: See the image attached below for the orbital diagram - Please leave a comment if you have any questions or need any clarifications. Magnesium is atomic ... Construct an orbital diagram to show the electron configuration for a neutral magnesium atom, Mg. Use the buttons at the top of the tool to add sublevels. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the Magnesium atom. In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for magnesium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
FREE Expert Solution. We’re being asked to construct the orbital diagram for Mg. For that, we first need to determine the electron configuration of Mg. Recall that for a neutral element, Atomic number = # of protons = # of electrons . The atomic number of Mg is 12 and since it’s a neutral element, this means Mg has 12 electrons.








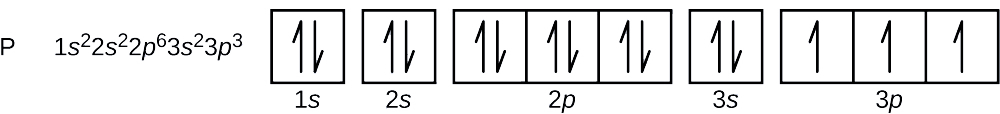












0 Response to "37 construct an orbital diagram to show the electron configuration for a neutral magnesium atom, mg."
Post a Comment