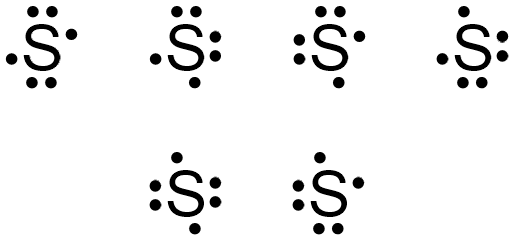
36 dot diagram for sulfur
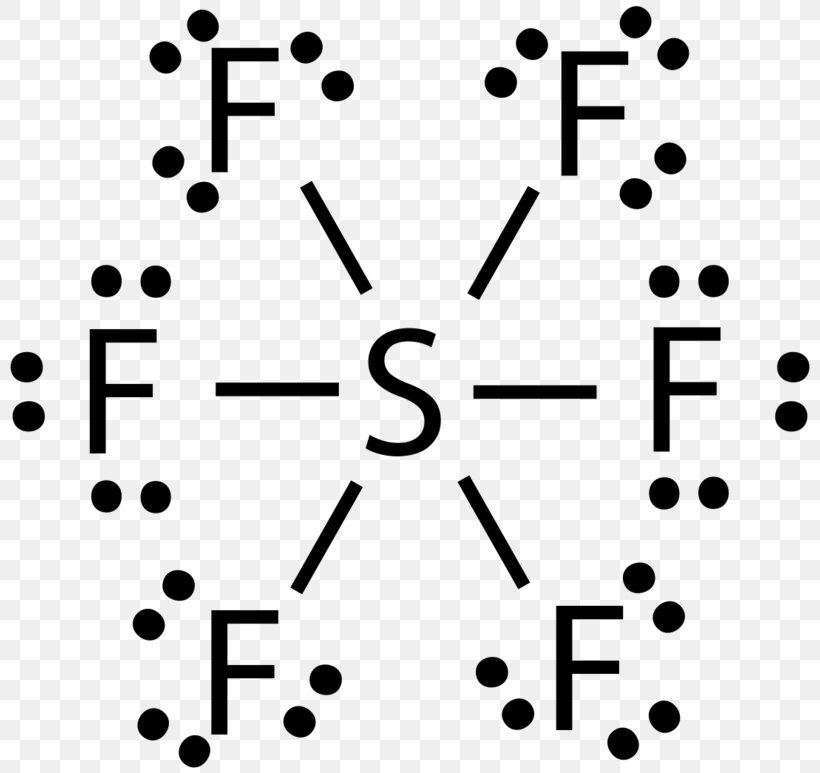
SF 6 (Sulfur hexafluoride) Lewis Structure. SF 6 (Sulfur hexafluoride) molecule contains one sulfur atom and six fluorine atoms. Lewis structure of SF 6 is given below. In SF 6 lewis structure, each fluorine atom has made single bonds with center sulfur atom. There are no lone pairs on sulfur atom and three lone pairs on each fluorine atom. In this tutorial, we will learn how to draw the lewis ...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Dot diagram for sulfur
Hazardous Substances Data Bank (HSDB) Sulfur dichloride is sold in technical grade with a chlorine content of 66 - 70%. The product contains about 72 - 80% SCl2, residual S2Cl2, and Cl2. Stabilized pure sulfur dichloride should contain minimum 98% SCl2.
The electronegativity value of the sulfur atom is 2.58, for a chlorine atom, it is 3.16. And for oxygen atoms, the electronegativity is 3.44. Hence, the sulfur atom is the least electronegative atom in the SOCl2 compound, therefore, we will put the sulfur atom at a central position in the lewis diagram.
Lewis dot structure for cas. October 22, 2021 thanh. 2. Draw the Lewis structures for the formation of CaS from the calcium and sulfur atoms (3 pts) 2.
Dot diagram for sulfur.
Dec 22, 2021 · Lewis Dot Diagram For Sulfur – Atkinsjewelry from hi-static.z-dn.net. Metallic elements, to the left of the staircase dividing line, tend to loose one or more electrons and form ions. In total 24 electrons to be drawn ( or 12 pairs. The total number of valence electrons for s is 6 (sulfur is also in the 6th column of the periodic table).
Below is the image of the lewis dot structure of Silicon and Sulfur separately. Now let us study the steps involved to draw the Lewis structure of Silicon disulfide (SiS2): Step 1 : Note down the total number of valence electrons available to draw one molecule of silicon disulfide : It is 16 as 4 are coming from silicon atom and 6 are coming ...
A step-by-step explanation of how to draw the S2- Lewis Dot Structure.For the S2- Lewis structure use the periodic table to find the total number of valence ...
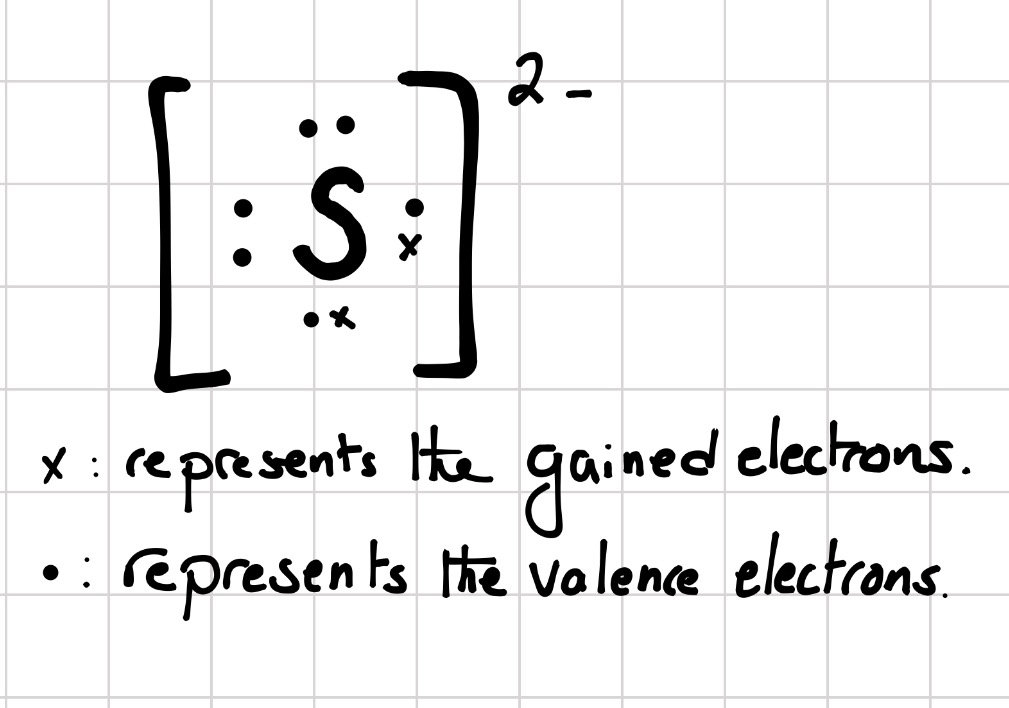
Lewis dot structure of Nitride ion. Now let us try Lewis dot structure of Sulfide ion ( S 2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). [Ne]4s 2 4p 6. Valence electrons are 8 (2 in 3s and 6 in 3p) Lewis dot structure of sulfide ion
A step-by-step explanation of how to draw the SI2 Lewis Dot Structure.For the SI2 structure use the periodic table to find the total number of valence electr...
Skill Sheet 16.1: Dot Diagrams Dot Diagram Element Sulfur Part 1 answers: Element Chemical Symb 01 Potassium Nitrogen Carbon Beryllium Neon Part 2 answers: Total El e s No. of Valence El e s Chemical Symbol Total Electrons No. of Valence Electrons D Page 27 of 44 Dot Diagram Chemical for Compound Formula Elements Dot Diagram for Each Element
Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4. Video: Sulfur Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ...
Sulfur dibromide (SBr2) lewis dot structure, molecular geometry, bond angle, polar or nonpolar Home > Chemistry Article > SBr2 lewis structure and its molecular geometry Sulfur dibromide is a toxic gas with the chemical formula SBr2.
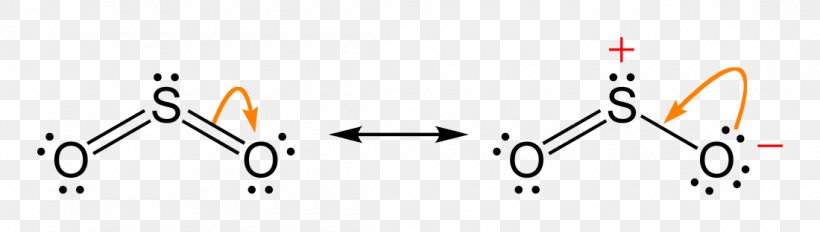
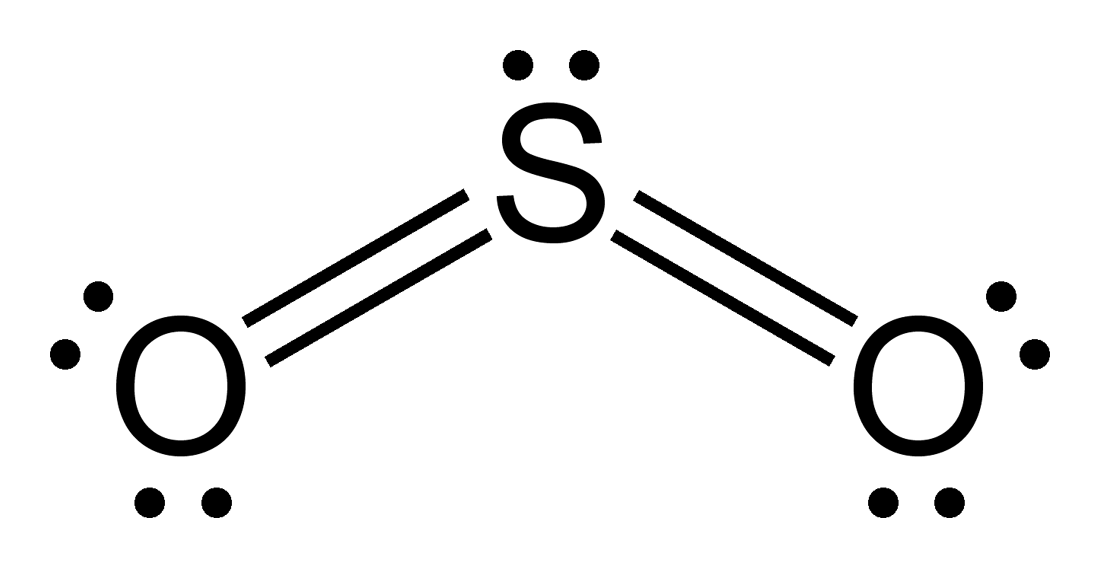
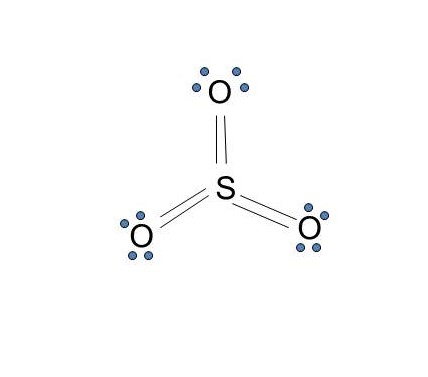
The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
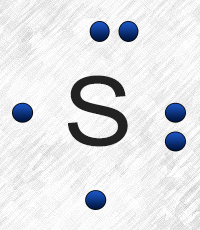
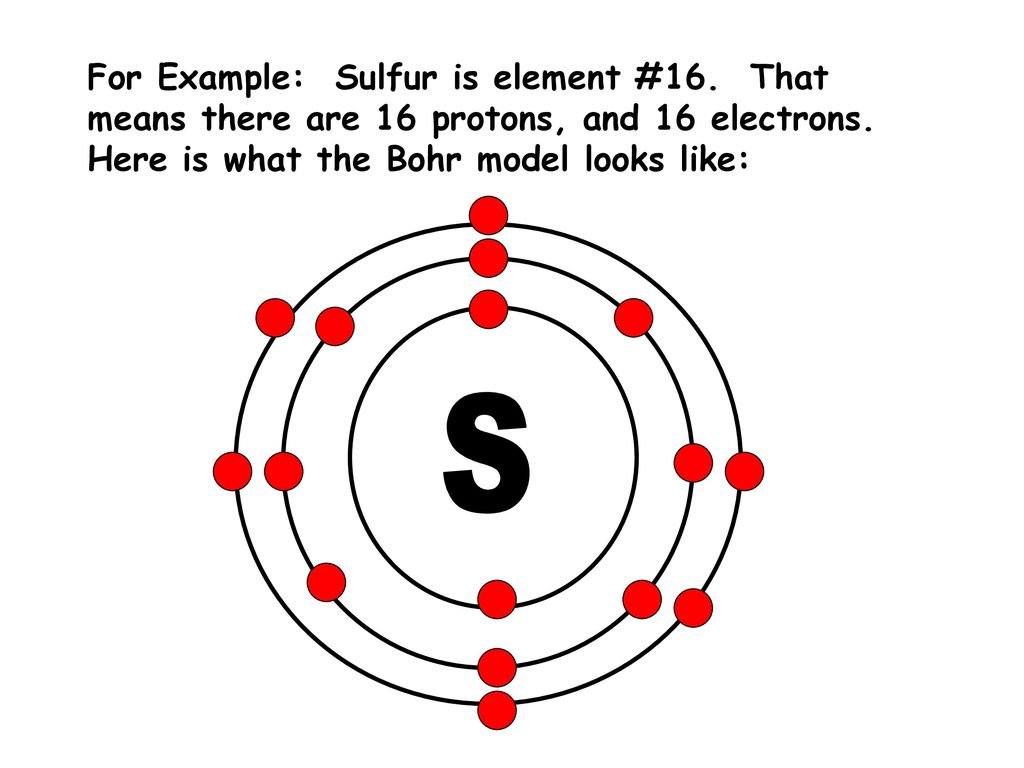
Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Sulfur, we got to know, it has 6 valence electrons. So, just represent the 6 valence electrons around the Sulfur atom as a dot. The electron configuration of Sulfur
Sulfur (in British English, sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent, and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Is so2 bent or linear?
Steps to draw electron dot structure or lewis structure of SCl2. Step 1: First you should count the total number of valence electrons in SCl2. For knowing valence electron you should know the group number of its compound. So, by observing the periodic table we know sulfur belongs to the 16th group and chlorine belongs to the 17th group.
Jun 18, 2019 · Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
Sulfur (S) has six valence electrons. A Lewis dot structure of around 'S' would have two dots on two sides, and one single on each of the remaining. It would look be drawn as: .. .S : .
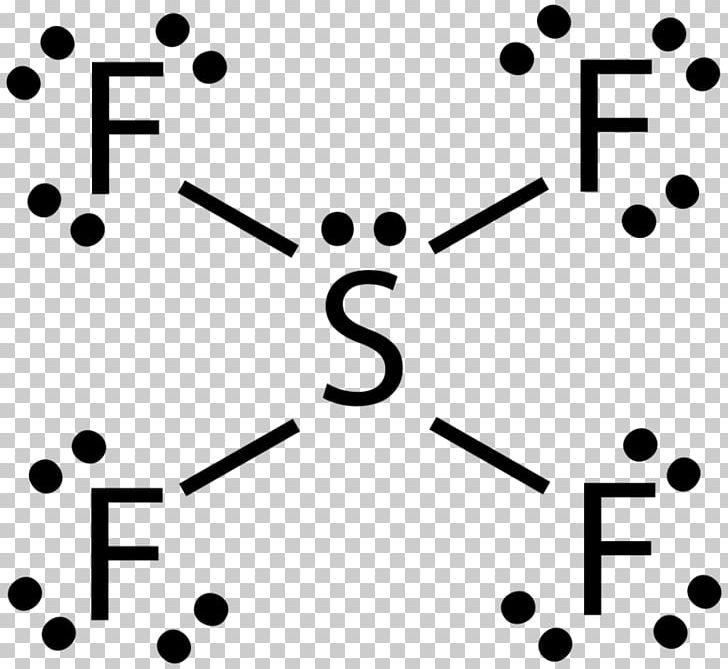
A step-by-step explanation of how to draw the SF4 Lewis Dot Structure (Sulfur tetrafluoride).For the SF4 structure use the periodic table to find the total n...
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access to the . Here are the steps I follow when drawing a Lewis structure. > 1.
Sulfur difluoride (SF2) lewis dot structure, molecular geometry, bond angle, polar or nonpolar Home > Chemistry Article > SF2 lewis structure and its molecular geometry Sulfur difluoride has a molar mass of 70.062 g/mol, it is highly unstable.
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. It has a chemical formula of SF 2 and can be generated by the reaction of Sulphur Dioxide and Potassium Fluoride or Mercury Fluoride. In this blog post, we will look at the Lewis dot structure of SF 2, its molecular geometry and shape.
Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na + ion, we note that the Na atom has a single valence electron in its Lewis diagram ...
Electron dot diagram for sulfur? The electron dot diagram for a lone uncharged Sulfur particle is an S with 6 electrons arranged around it (2 orbitals with 2 electrons and 2 orbitals with 1). How...
A step-by-step explanation of how to draw the Lewis dot structure for S (Sulfur). I show you where Sulfur is on the periodic table and how to determine how ...
Lewis Dot Structure For Lithium And Sulfur, Solfuro di litio Wikipedia, Multimedia: Represent Bonding with Lewis Dot Diagrams, Chemistry 101 : Chap 8 PowerPoint Presentation, 31 Lewis Dot Diagram Worksheet Pdf Free Wiring Diagram
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2. Viewing Notes: The Lewis structure for ...




















0 Response to "36 dot diagram for sulfur"
Post a Comment