35 energy vs reaction coordinate diagram
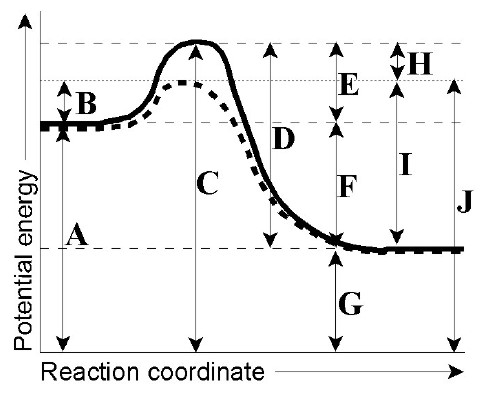
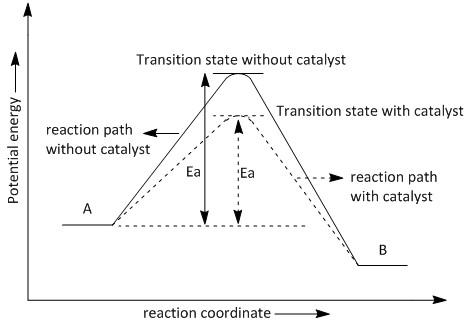
Diagram of a catalytic reaction, showing the energy niveau depending on the reaction coordinate. For a catalysed reaction, the activation energy is lower. In chemistry , a reaction coordinate [1] is an abstract one-dimensional coordinate which represents progress along a reaction pathway. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right the progress of the reaction from starting compounds to final products.
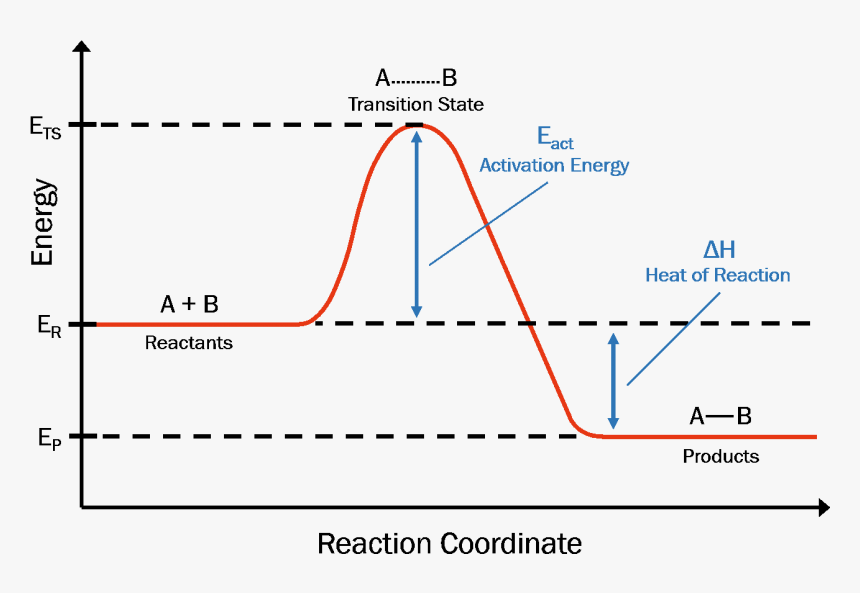
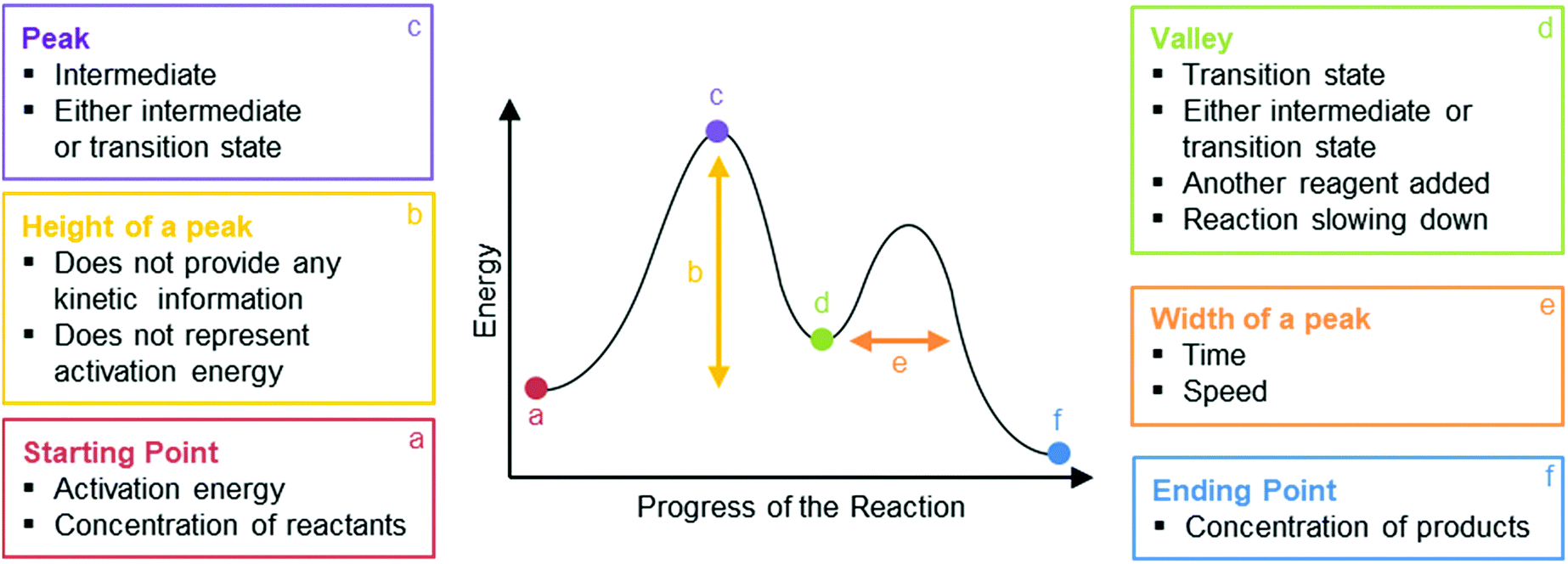
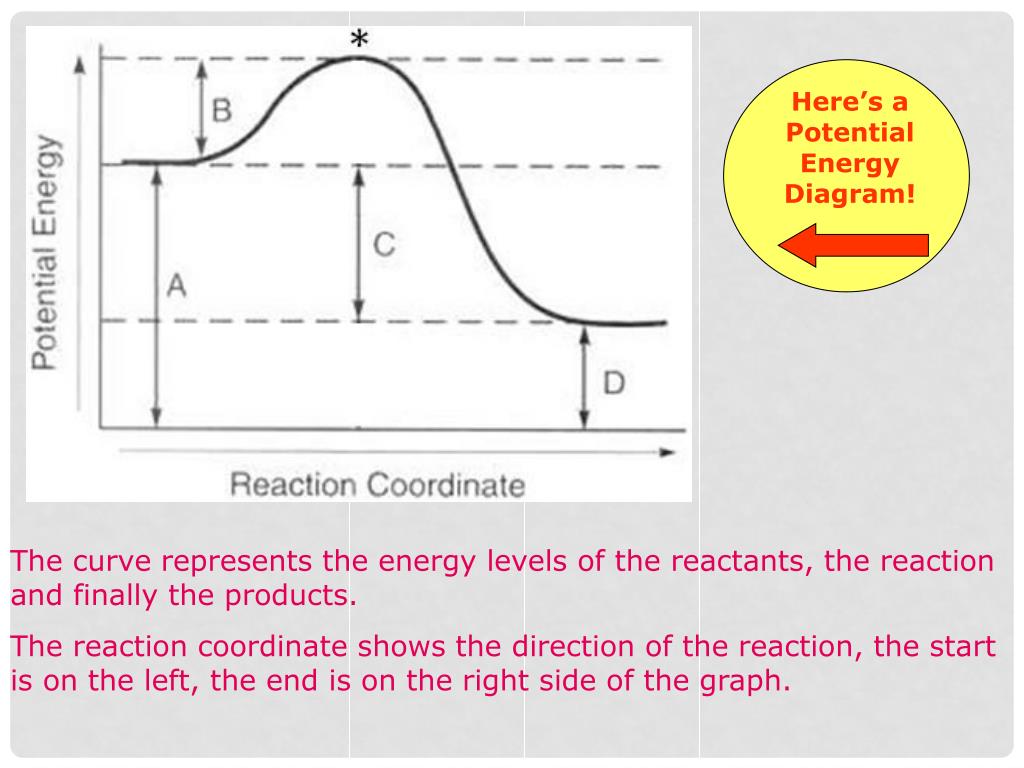
A reaction energy diagram (Figure 1) is presented on the chalk board (complete with axes labeled: potential energy vs. reaction coordinate (or reaction progress)). The activation energy, Ea, (the change in energy from reactants to the top of the "hill") is labeled. The students are taught that the species at the top of
Energy vs reaction coordinate diagram
Indian Institute of Science. I am adding here a MATLAB code for drawing free energy reaction profile. This is just for plotting del Grxn vs reaction coordinate. (A small change is needed to ... This type of graph is called a reaction coordinate diagram. Gibbs energy is plotted on the y-axis, and the x-axis in arbitrary units shows the progress of a reaction. In the case of an exergonic reaction, the figure on the left indicates two key things: (1) the difference between the free energy of the reactants and products is negative and (2 ... Consider the Potential Energy vs. Reaction Coordinate diagram shown to the right. For each of the following questions, circle the best answer. If the temperature is lowered, i) equilibrium will shift toward reactants. ii) equilibrium will shift toward products. iii) no change in equilibrium position
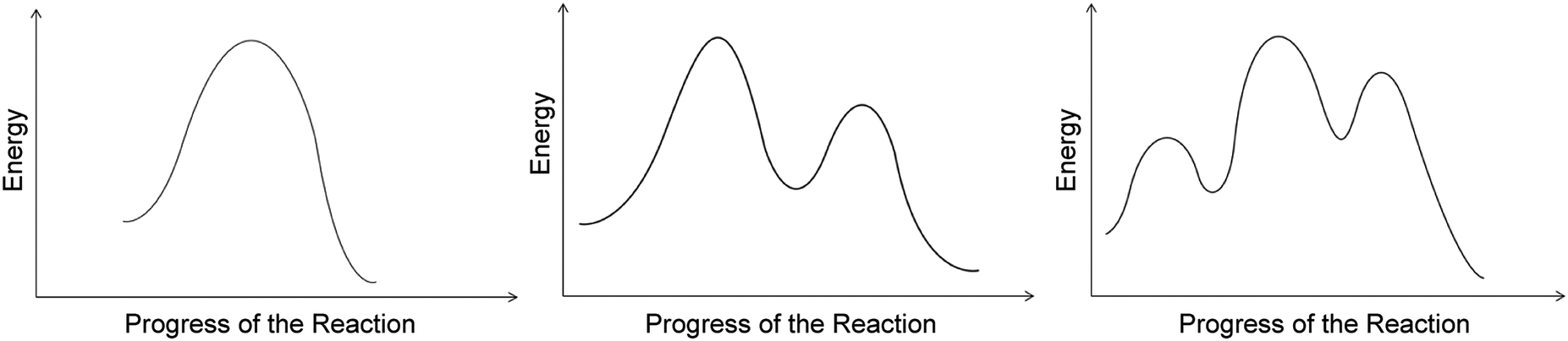
Energy vs reaction coordinate diagram. Fig. 1.1: Free energy vs Reaction coordinate diagram for above reaction 1.1.2 Definition of Thermodynamic Terms: 1.1.2.1 System: It is defined as any portion of the universe or the quantity of matter that Despite its apparent simplicity, this energy diagram conveys some very important ideas about the thermodynamics and kinetics of the reaction. Reaction coordinate diagrams. The intrinsic reaction coordinate (IRC), derived from the potential energy surface, is a parametric curve that connects two energy minima in the direction that traverses the minimum energy barrier (or shallowest ascent) passing through one or more saddle point(s). However, in reality if reacting species attains enough energy it may deviate from the IRC to some extent. A reaction coordinate diagram is a graph that plots energy versus reaction progress. The amount of energy that needs to be added is called the activation energy, which is the point where the line ...
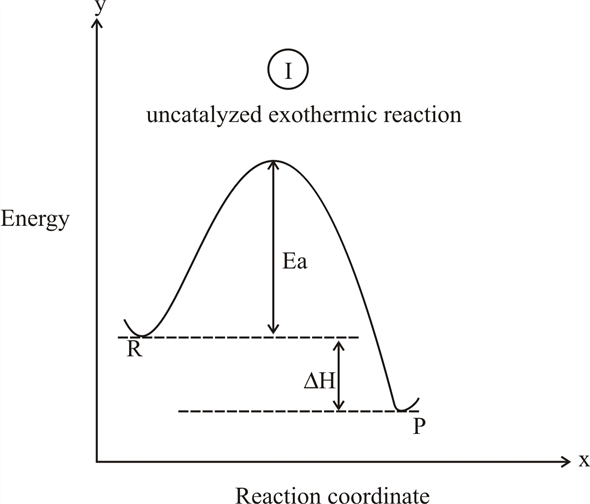
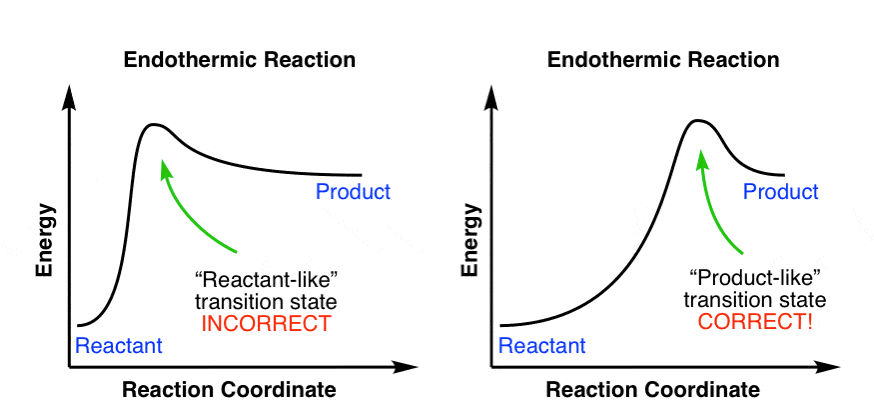
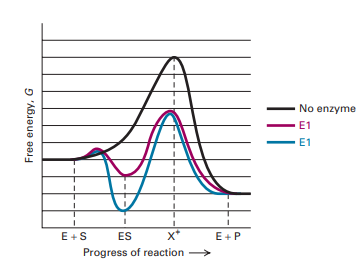
On this diagram we see: the x-axis that is a reaction coordinate: a loosely defined term meaning the reaction progress in the general direction from the starting materials or reagents (SM) to the products (Pr). the energy curve describing the energy states of the components at a certain point in the reaction. Energy diagrams for endothermic and exothermic reactions. This is shown in an Energy vs Reaction Coordinate plot. useful in showing: 1) whether a reaction is an exo- or endothermic process, 2) which reaction Exothermic reactions will have products that are lower in energy than the reactants.Endothermic Reaction Energy Diagram - 62 Unique Figure ... Problem: Consider the Potential Energy vs. Reaction Coordinate diagram shown to the right. For each of the following questions, circle the best answer.If a catalyst is added, i) ΔG° for the reaction increasesii) ΔG° for the reaction decreasesiii) ΔG° for the reaction equals 0 iv) ΔG° for the reaction in unchanged This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the free energy of the products is greater than the free energy of the reactants. Label all quantities as in Model 1. Products a(fwd) Reactants Reaction Coordinate Using your diagram from Exercise 3, identify which reaction (forward or reverse) has the larcer The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants. Diels-Alder Reaction: A Reaction with High Atom Economy Study Questions 1) Given the following reaction sequence and information, draw an energy diagram (Energy vs Reaction Coordinate) illustrating the major energetic features of the following reaction. Answer: 2) Provide the products for the following Diels-Alder reactions.
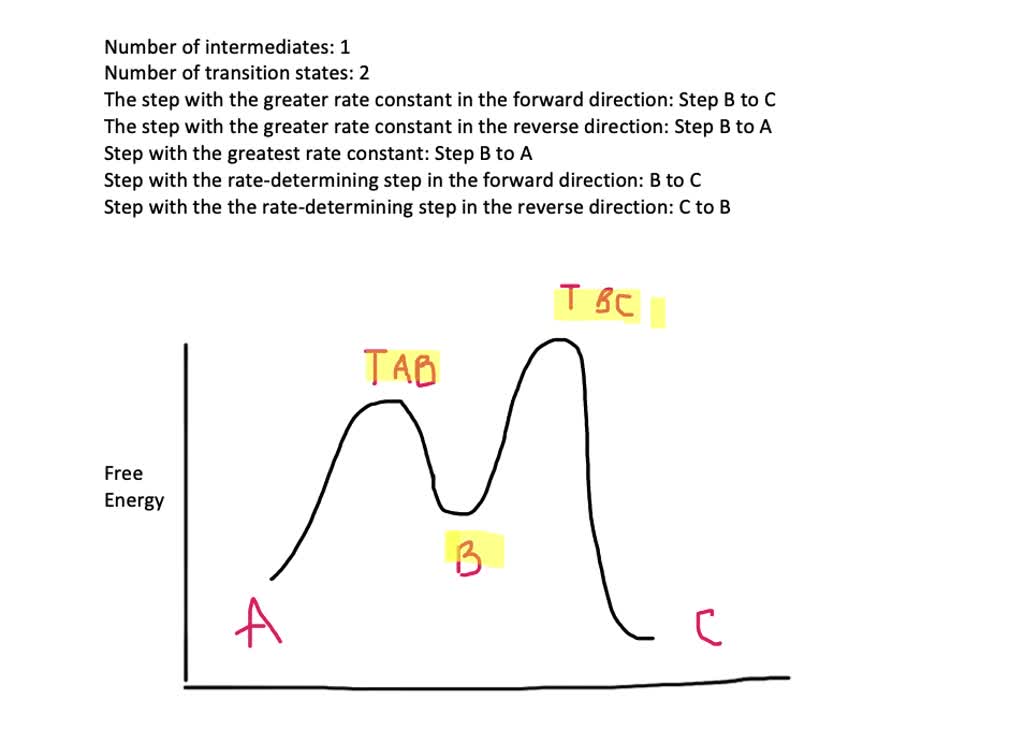
Select all the statements that correctly describe the reaction coordinate vs. energy diagram for an SN1 reaction. A. The energy diagram shows two energy maxima corresponding to the 2 transition states B. The carbocation intermediate is more stable than the starting material. C.
1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction
The difference in energy between the reactants and the point of maximum energy ... Now that we've seen the basic features of a reaction coordinate diagram, ...
Reactants Products + Energy. Draw a reaction coordinate diagram for this reaction as above but add the activation energy, E a, for the catalyzed reaction on the appropriate curve in this diagram and label it. This is a bit more subtle since .Types of catalysts (article) | Kinetics | Khan AcademySection The Rate of a Reaction ...
The. Question: Use the free energy versus reaction coordinate diagram to answer the three questions below. How many transition states are there? Which of the following represents Delta G for this reaction? A B E The difference between A and B The difference between A and E Which of the following represents the free energy of activation for this ...
Reaction potential energy diagrams are graphs that show the energy of a process as a function of the extent to which that process has occurred. As these are graphs showing mathematical functions, there must be a numerical coordinate axis that shows the independent variable. This coordinate is called the reaction coordinate, and it reflects the ...
Energy Surfaces vs. Reaction Coordinate Diagrams Something that cannot be represented in the three dimensional energy surface below is the differentially populated vibrational modes of the molecules Reaction coordinate diagrams are used to simplify the situation, they represent a composite
Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol-1. Barriers are measured in energy per mole (typically kJ mol-1).
1i. Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the energy of the products is greater than the energy of the reactants. Label all quantities as per Fig. 1. 1j. Using your diagram from exercise 1i, identify which reaction (forward or reverse has the
Draw a reaction coordinate vs. potential energy diagram for a reaction system where the KINETIC PRODUCT is the MORE stable of two possible products. Label on the reaction curves where the kinetic and thermodynamic products, respecively, are located. 2. Draw a reaction coordinate vs. potential energy diagram for a reaction
Thermodynamics vs Kinetics Overview A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be
1i. Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the energy of the products is greater than the energy of the reactants. Label all quantities as per Fig. 1. See diagram (3) in sample exercise 14.10 on pg 595 of Brown and LeMay, 11th ed.
Consider the Potential Energy vs. Reaction Coordinate diagram shown to the right. For each of the following questions, circle the best answer. If the temperature is lowered, i) equilibrium will shift toward reactants. ii) equilibrium will shift toward products. iii) no change in equilibrium position
This type of graph is called a reaction coordinate diagram. Gibbs energy is plotted on the y-axis, and the x-axis in arbitrary units shows the progress of a reaction. In the case of an exergonic reaction, the figure on the left indicates two key things: (1) the difference between the free energy of the reactants and products is negative and (2 ...
Indian Institute of Science. I am adding here a MATLAB code for drawing free energy reaction profile. This is just for plotting del Grxn vs reaction coordinate. (A small change is needed to ...






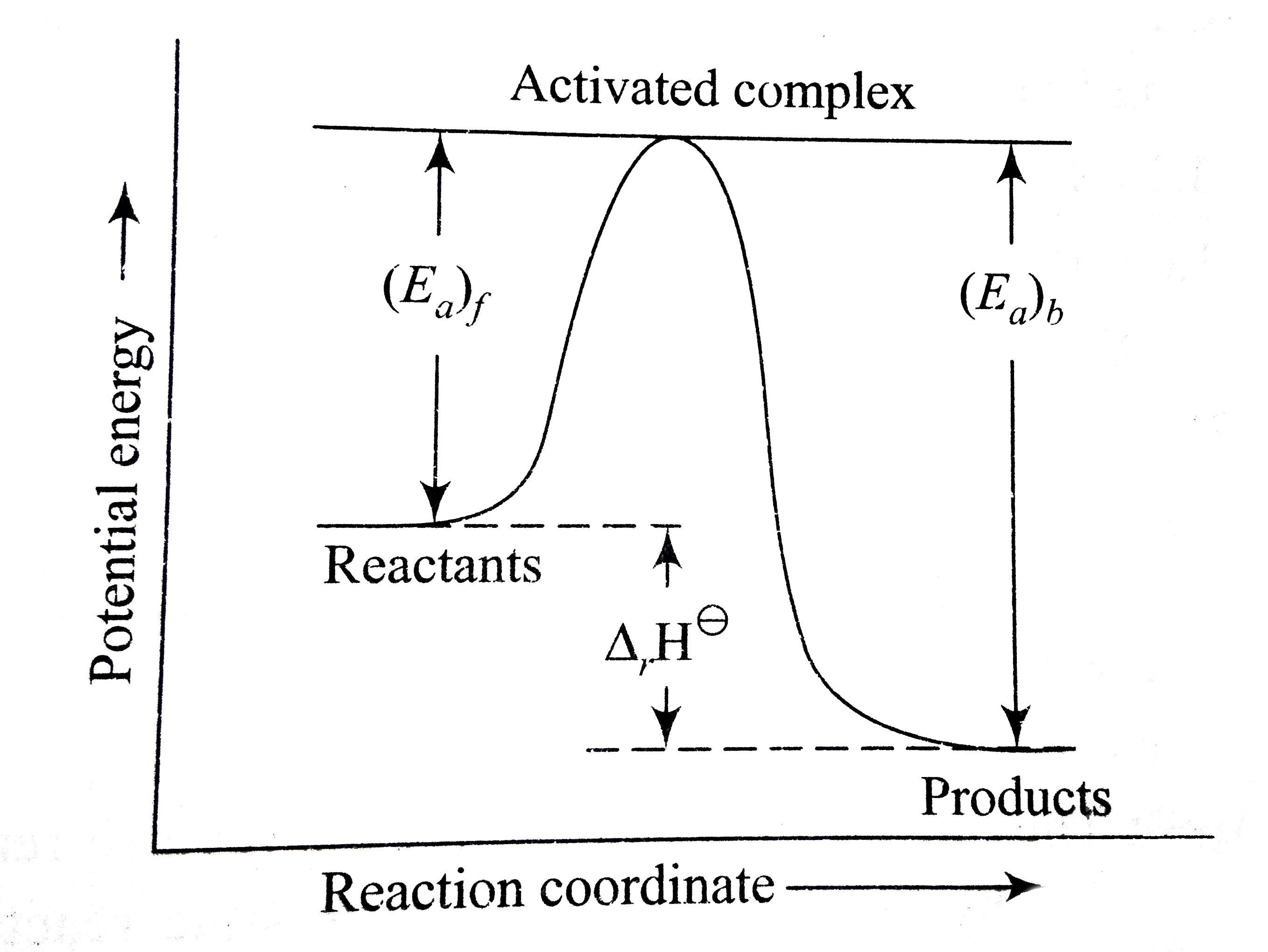











0 Response to "35 energy vs reaction coordinate diagram"
Post a Comment