35 ch4 electron dot diagram
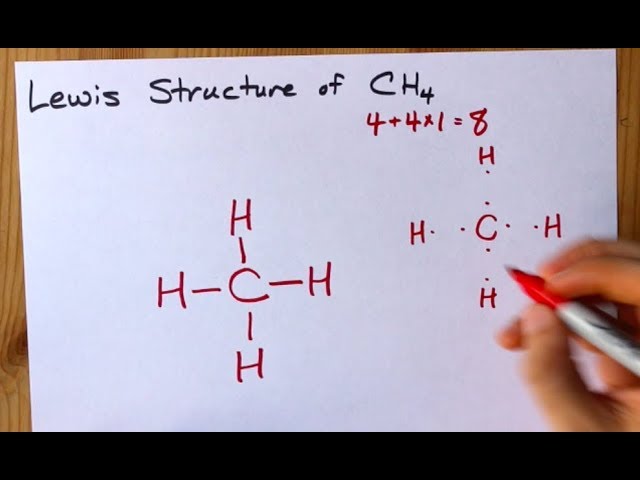
For CH4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH4 (named methane) requires only single bonds. It's one of the easier ...Oct 27, 2016 · Uploaded by Wayne Breslyn
The Lewis structure is the pictorial representation of the valence electrons that participate in the bond formation as well as the ones that don't. Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in ...
Methane (CH4) -. Methane is 25 times stronger than carbon dioxide in terms of its global warming potential. Nitrous oxide (N2O) -. Dichlorodifluoromethane (CCl2F2) -. Chlorodifluoromethane (CHClF2) -. Tetrafluoromethane (CF4) -. Hexafluoroethane (C2F6) -. What is the Lewis dot structure of NO2-? NO2 (Nitrogen Dioxide) Lewis Dot Structure.
Ch4 electron dot diagram
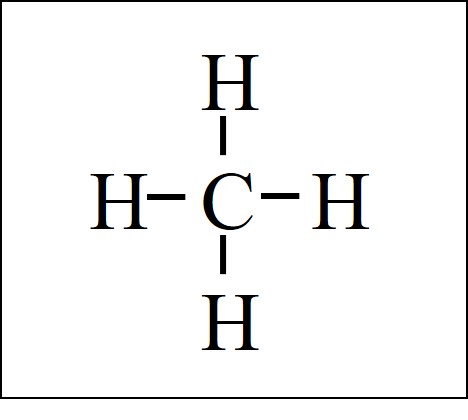
The lines represent bonds. This is the final Lewis Dot Diagram. The pairs of dots represent shared electrons and you can see that the Carbon ...1 answer · 1 vote: I will explain this with pictures, and some captions. This is just the five atoms in ...
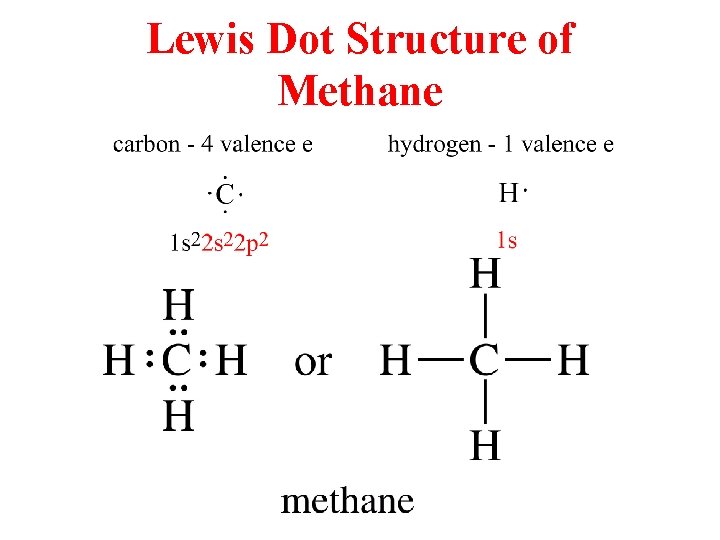
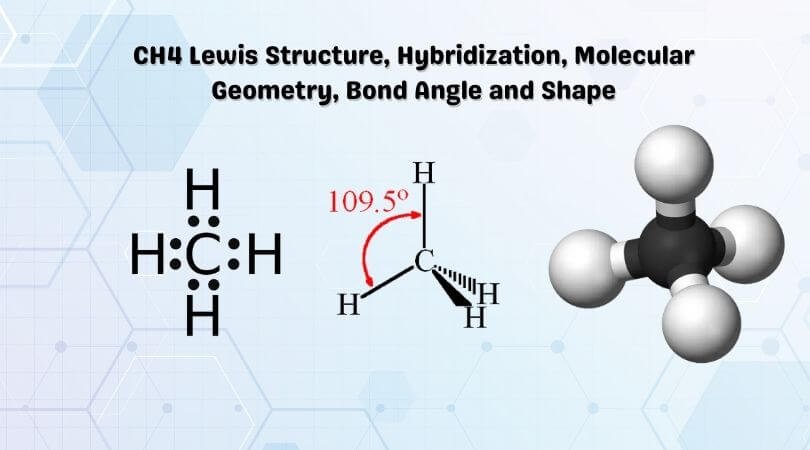
What is the electron dot structure for CH4? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
Total number of valence electrons for CH4 - 4 + 4 = 8 Thus there eight valence electrons for Methane. CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms' bond formations.
Ch4 electron dot diagram.
A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4's and CH4 MO diagram looks like.
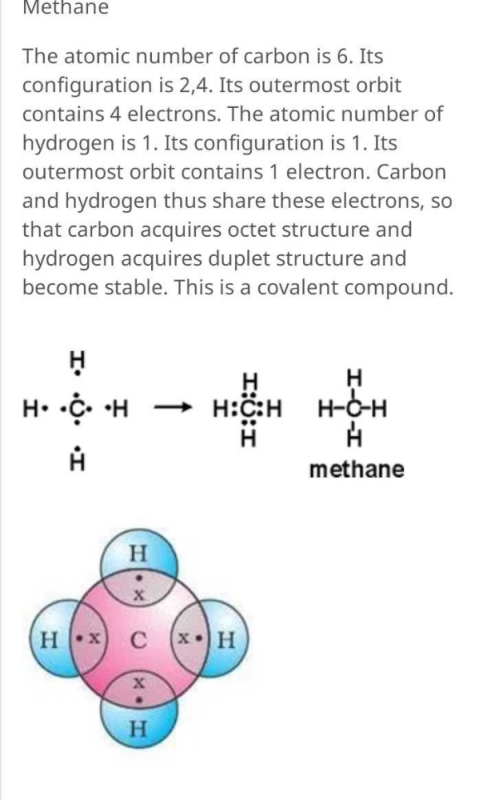
1 answerMethane. The atomic number of carbon is 6. Its configuration is 2,4. Its outermost orbit contains 4 electrons. The atomic number of hydrogen is 1.
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.
To draw the lewis Dot structure of CH₄(methane), we have to find out the valence electrons of carbon and hydrogen first.We express valence electrons as dots in lewis dot structure. How … In fact the molar mass of methane is so minuscule that it is sometimes mentioned as a possible lifting gas because its density is less than that. is methane ionic or covalent, _____4.
Ch4 dot diagram. The lewis dot structure for ch4 is shown above. 8 octet electrons x 1 atom 8 octet electrons h. Note that hydrogen atoms always go on the outside of a lewis dot structure. The electrons are arranged in four pairs representing four covalent bonds. 2 octet electrons x 4 may 03 a step by step explanation of how to draw the lewis ...
15 Ch4O Lewis Structure. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. The xe atom is attached to all four oxygen atoms. Worksheet 1 - solutions A - CHEM 2400 Video 1-2…
Which of the following is/are not the condition(s) for Lewis dot structure? (i) Each bond is formed as a result of sharing of an electron pair between the atoms. (ii) From the two combining atoms only one atom contribute electron(s) to the shared pair. ... CH 4 (c) CO 2 (d) NO. Answer. D.
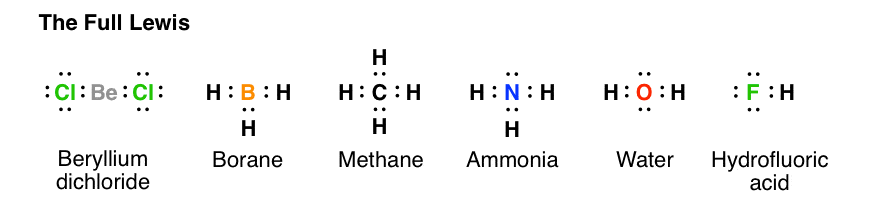
Lewis dot diagrams for the folowing a. Hydrogen (H 2) b. Water (H 2 O) c. Carbon dioxide (CO 2) d. Methane (CH 4) e. Lithium Fluoride (LiF) Answer: [Note: H atom in H 2 and Li atom in LiF attain the configuration of helium (a duplet of electrons).] Question B. Diagram for bonding in ethene with sp 2 Hybridisation. Answer: Question C. Lewis ...
The Lewis-dot-structures can be written by adopting the following steps: 1. The total number of electrons required for writing the structures is obtained by adding the valence electrons of the combining atoms. For example, in the CH 4 molecule, there are eight valence electrons available .for bonding (4 from carbon and 4 from the four hydrogen ...
The lewis structure of CH4 is drawn to fulfill the need of valence electron by every the atoms. Lewis structure of CH4 The lewis structure of carbon and hydrogen atom says- to form a solitary CH4 molecule, a complete of eight valence electrons take part in the common bonding to fulfill the require of eight an ext valence electrons.
Draw the electron-dot structures of the following compounds and state the type of bonding in each case: (i) KCl. (ii) NH 3. (iii) CaO. (iv) N 2. (v) CaCl 2. Class 10th. Chemistry. Lakhmir Singh and Manjit Kaur.
Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source.
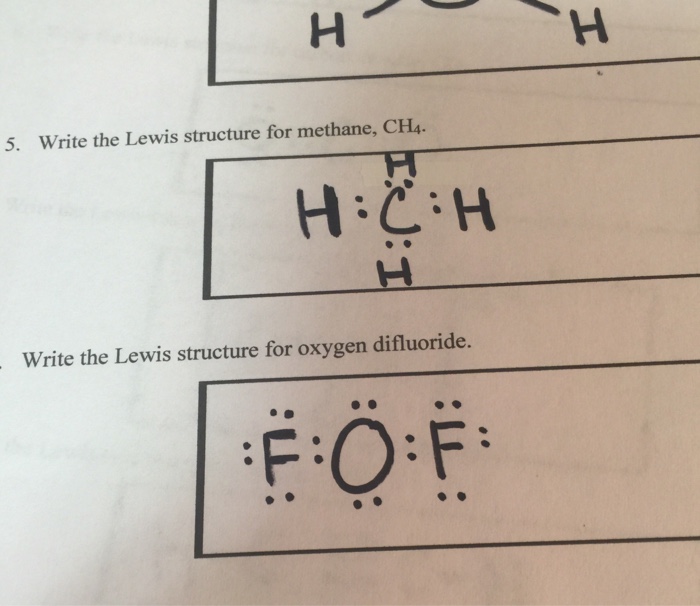
The electron dot structure of OF 2 is Thus the central atom (O-atom) has 4 pairs of electrons (2 bond pairs and 2 lone pairs). Hence oxygen in OF 2 is sp 3 hybridised and the molecule is v-shaped oxidation state of F = - 1, oxidation state of O = + 2.
What is the Lewis dot structure for CCl4? Lewis Dot of Carbon TetraChloride CCl4 TetraChloromethane. In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds. Because of this symmetrical geometry, CCl 4 is non-polar.
The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons.
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots( ) but a lone pair of two electrons is shown by dots[ ].
This is just the five atoms in CH4, or Methane. Draw the electron dot structure of methane thiol, identifying the correct VSEPR shape of the molecule. Using electron-dot diagrams which show only the outermost shell electrons show how a molecule of nitrogen N2 is formed from two nitrogen atoms. Required fields are marked *. Do not despair.
Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source.
Lewis Dot Structure for Compounds. Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows ...
CH4 Lewis structure Methane electron dot structure is that type of diagram where we show the total 8 valence electrons of CH4 as dots or dots and dashes -In Lewis structureit is common that a bonding pair of two electrons can be shown by dash - or dots but a lone pair of two electrons is shown by dots. A Estructura electrónica de Lewis.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electron s which for m 2 lone pairs. Since it is bonded to only one carbon atom, it must for m a double bond.. Answer (1 of 4): draw the lewis dot diagram for H2S.
The lewis structure of bef2 (figure 2) shows only two electron pairs around the. An example of a nonpolar molecule is ch4. Why is ch4 non polar from slidetodoc.com draw the lewis structure, then determine the shape of the molecule. In the ch4 lewis structure, there is a single bond between carbon and hydrogen atoms .
In CH4, the central atom may be carbon. Within the electron point structure, we represent the e-value of the element. Thus, the Carbon © electron (e) has 4 electrons within the point structure and one electron within the hydrogen (H) atom. C - H shares electrons to make one bond. CH4 Lewis Structure Lewis Structure for CH4 (Methane).
Lewis Structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.
Diagrama De Lewis Ch4 ch4 lewis dot diagram structure draw molecular estructura methane cross ã ã ã ã Electron formula î CH4î svg Wikibooks ch4 methane punkte svg lewis structure chemistry valence electrons ch wikipedia commons structures each carbon file wikimedia molecule octet draw dot methane ch4 lewis diagram electron structure ...
Solution · In CH4, the central atom is a carbon. In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its ...1 answer · Top answer: In CH4 , the central atom is a carbon.In electron dot structure we represent the valence electron of the element.Thus, Carbon has 4 electrons in its ...


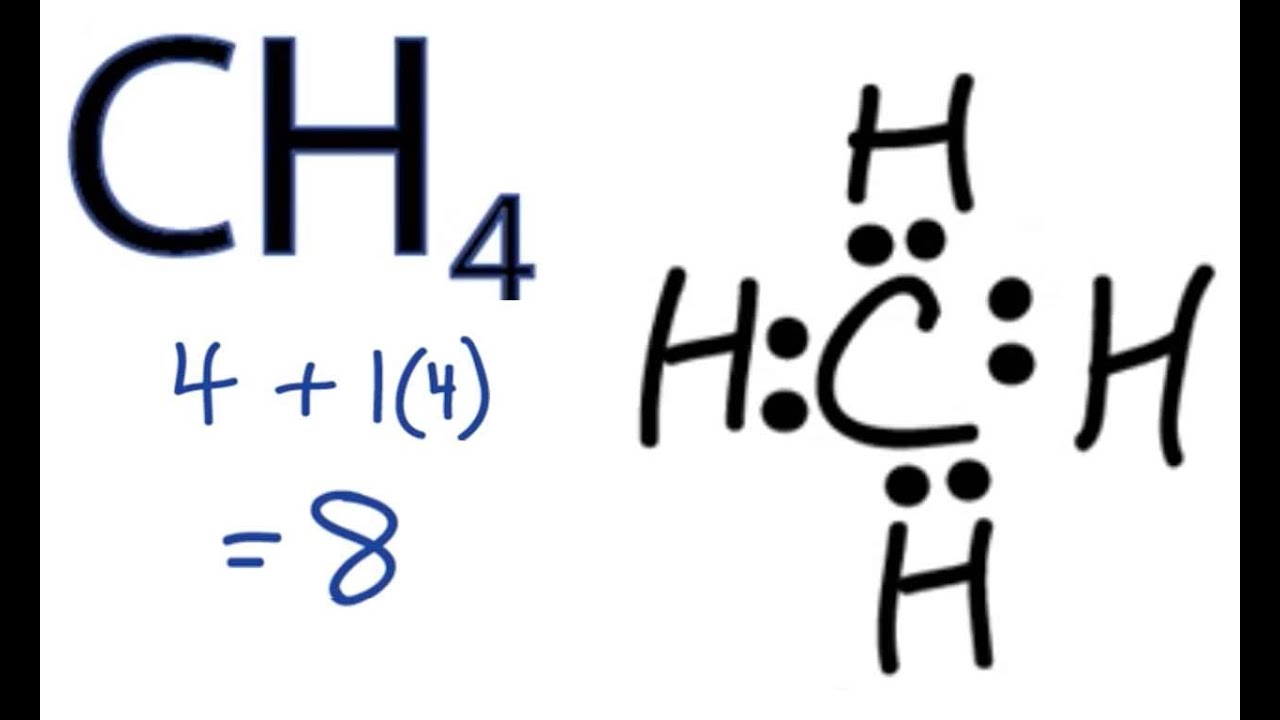














![Expert Verified] Draw electron dot structure of methane and ...](https://hi-static.z-dn.net/files/dbd/fbdbc86f5bd1bd2a51aa3e12c90747c6.jpg)






0 Response to "35 ch4 electron dot diagram"
Post a Comment