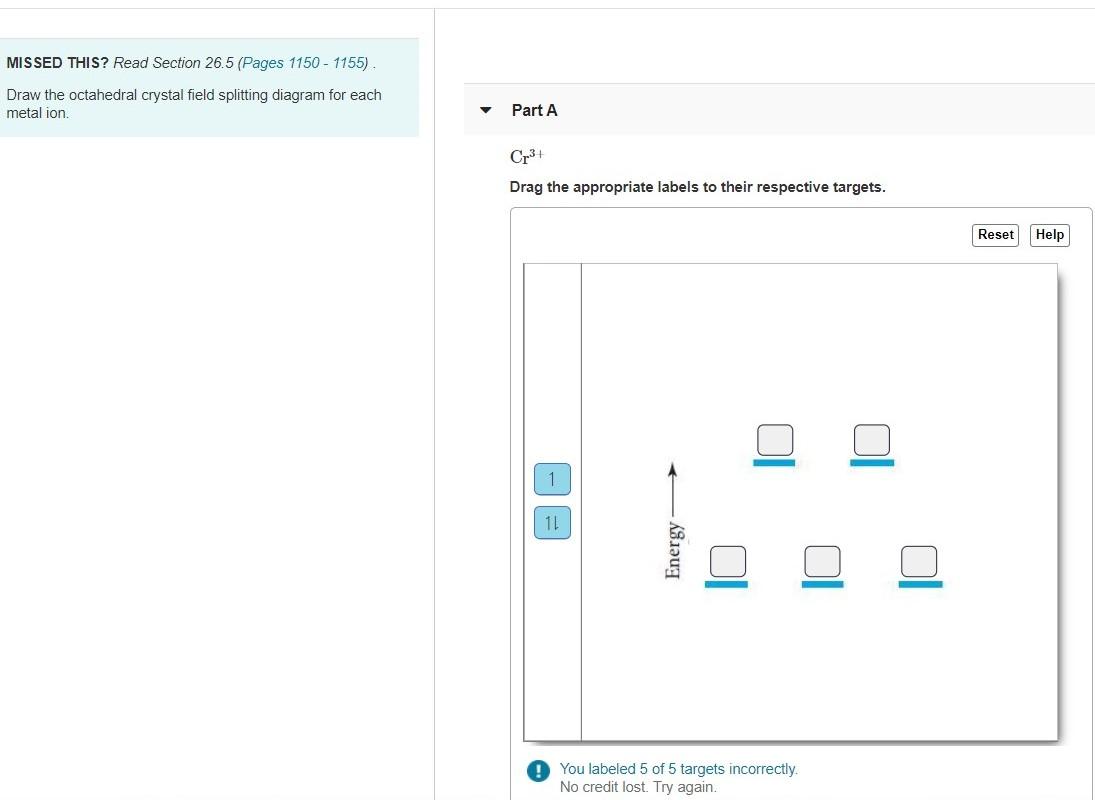
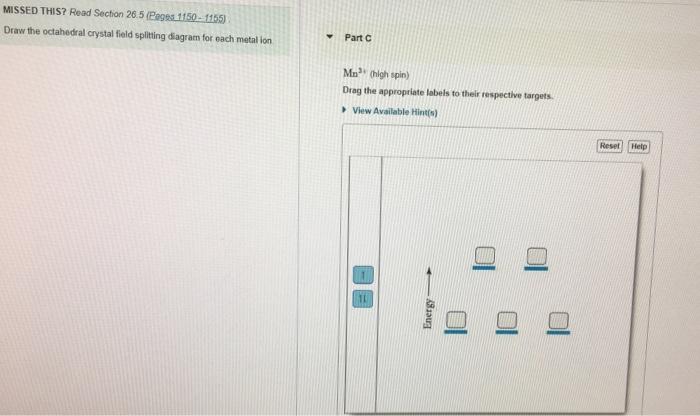
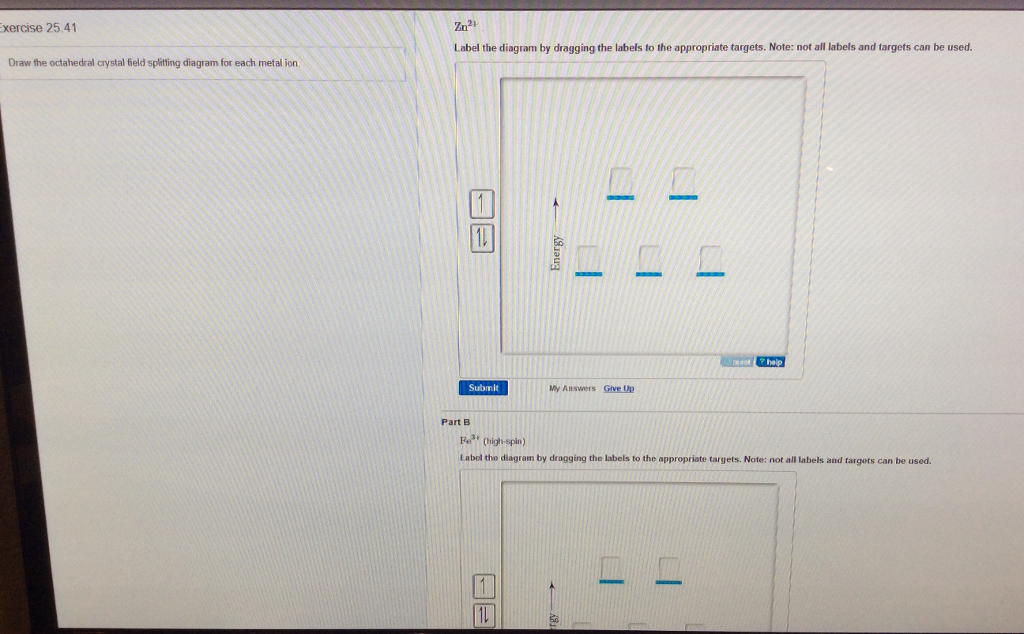
34 draw the octahedral crystal field splitting diagram for each metal ion.
4) Using crystal field theory, draw the crystal field d orbital energy level diagram for each of the following complexes by assigning electrons to 3d orbitals. [Co(en)2]2+ (square planar) [FeF6]3- (octahedral) 5) For the following complex, draw an orbital diagram for the isolated metal ion.
Overview of crystal field theory. According to crystal field theory, the interaction between a transition metal and ligands arises from the attraction between the positively charged metal cation and the negative charge on the non-bonding electrons of the ligand. The theory is developed by considering energy changes of the five degenerate d-orbitals upon being surrounded by an array of point ...
For octahedral complexes, crystal field splitting is denoted by . We find that the square planar complexes have the greatest crystal field splitting ligand field (left diagram) and the tetrahedral field (right diagram).D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with ...

Draw the octahedral crystal field splitting diagram for each metal ion.
All right. This question coverage. Crystal field theory and not cathedral field splitting diagrams. And to review uh we can split our five D orbital's shown he…
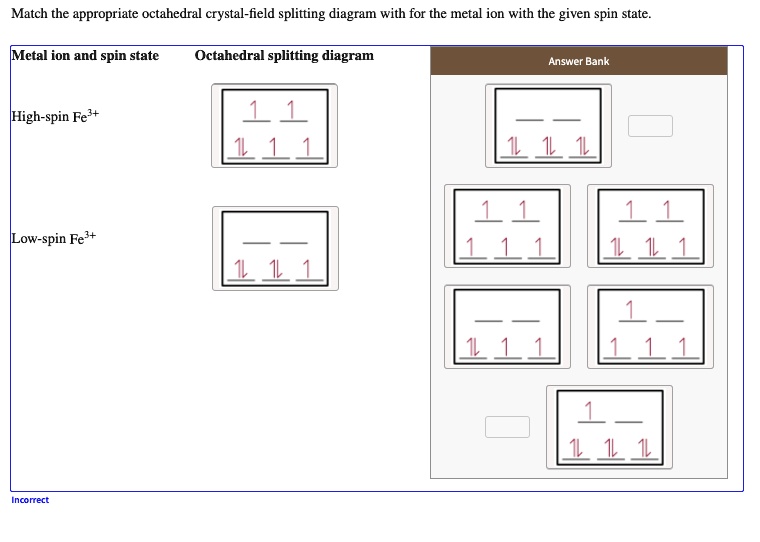
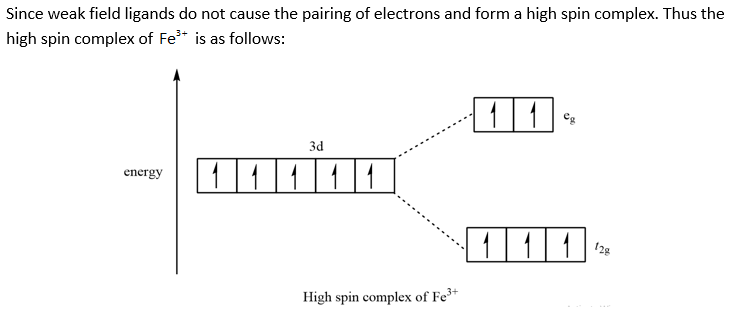
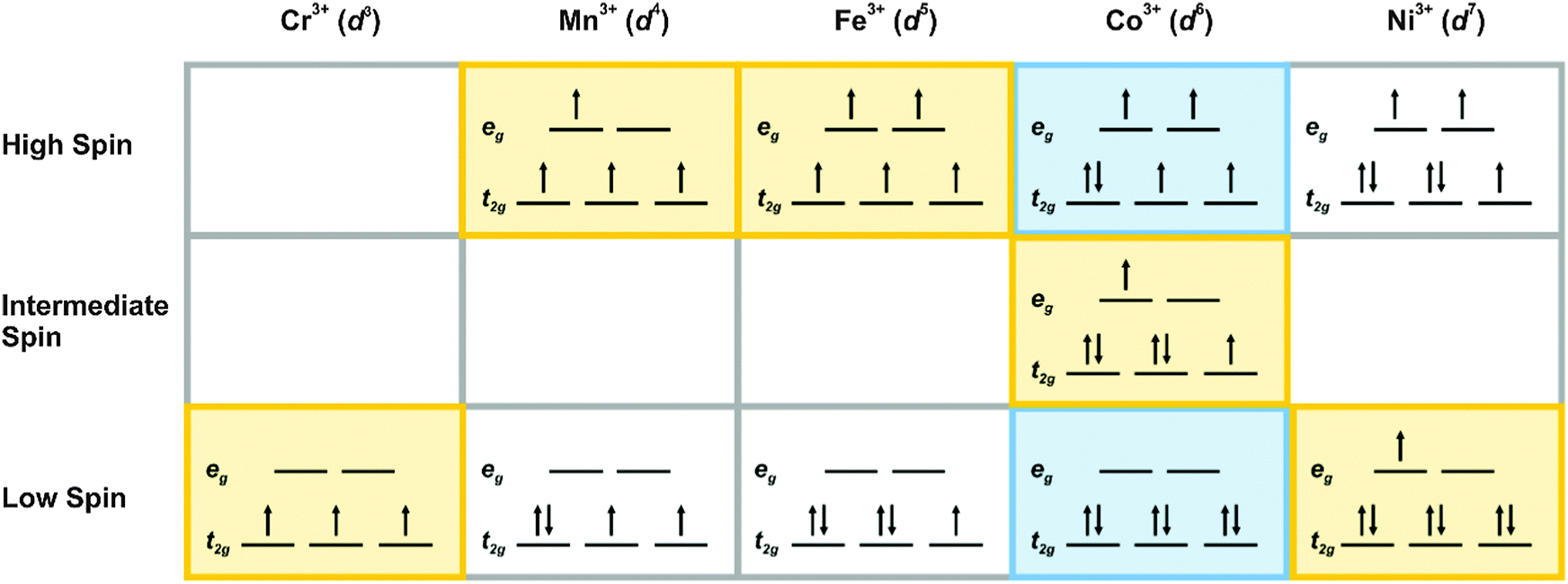
Let's calculate the crystal field Stabilization Energy for high-spin and low-spin octahedral cobalt(III) in terms of the splitting energy and the pairing energy. Solution. Cobalt(III) is a d 6 metal ion. The diagrams for the free ion, high-spin cobalt(III), and low-spin cobalt(III) are shown below: The CFSE for the high-spin case is
The d x 2-y 2 and d z 2 orbitals on the metal ion at the center of the cube lie between the ligands, and the d xy, d xz, and d yz orbitals point toward the ligands. As a result, the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex.
Draw the octahedral crystal field splitting diagram for each metal ion..
A Single d-Electron in an Octahedral Field The Negatively Charged Ligands Produce an Electric Field+Potential The Field Interacts with the d-Electrons on the Metal (Repulsion) The Interaction is NOT Equal for All Five d-Orbitals 1. The Spherical Symmetry of the Free Ions is Lifted 2. The d-Orbitals Split in Energy 3.
Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected.
For each of the following metals, write the electronic configuration of the atom and its 2+ ion (a) Mn, (b) Ru, (c) Rh. Draw the crystal-field energy-level diagram for the d orbitals of an octahedral complex, and show the placement of the d electrons for each 2+ km, assuming a strong-field complex. How many impaired electrons are there in each case ...
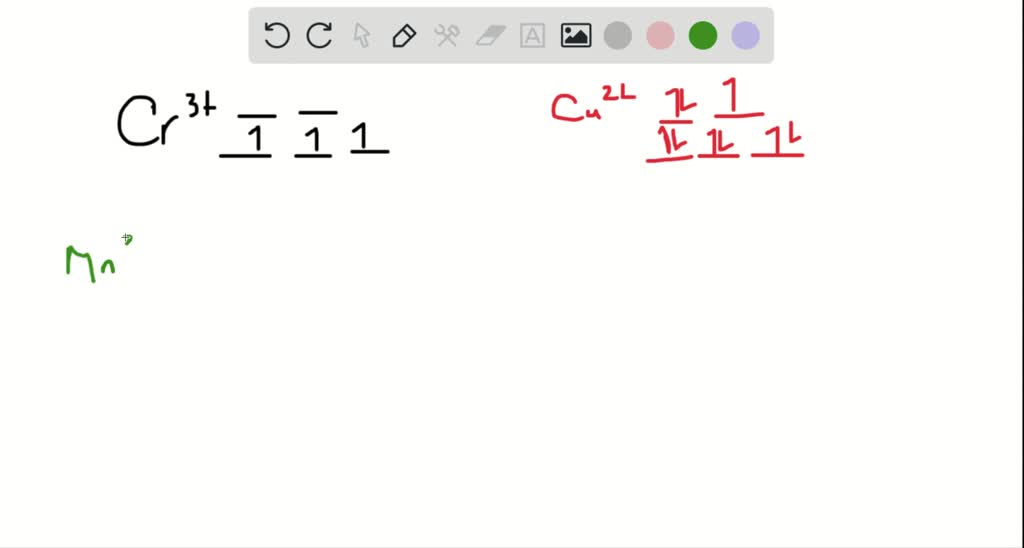
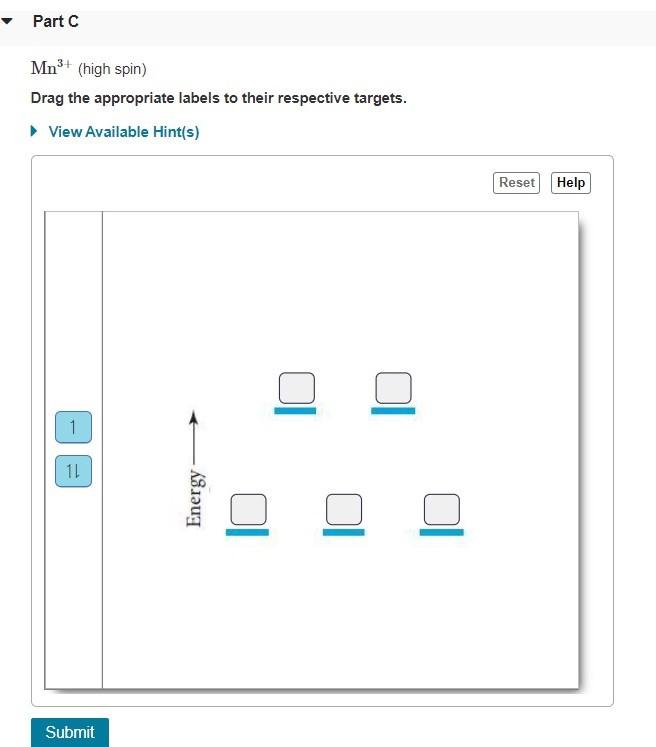
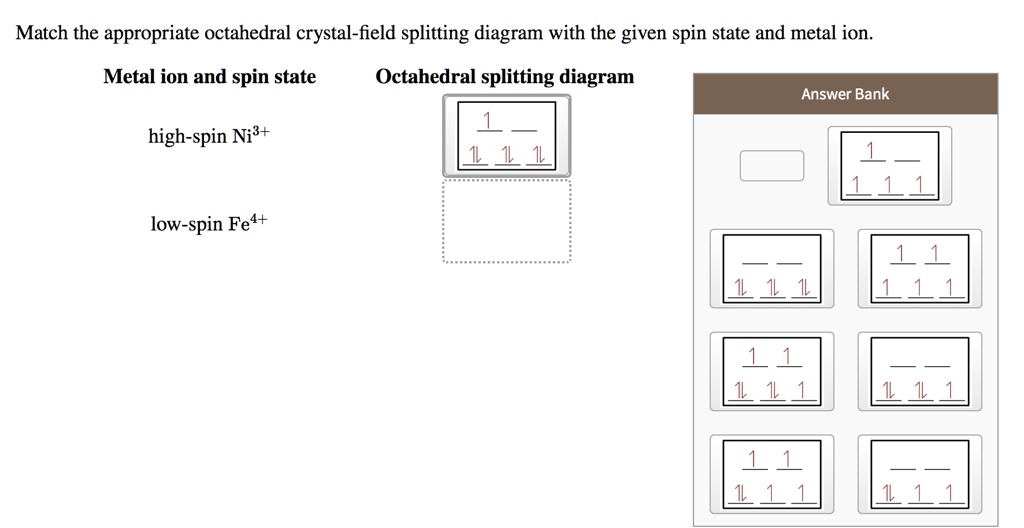
Draw the octahedral crystal field splitting diagram for each metal ion. Cr3+ Cu2+ Mn3+ (high-spin) Mn3+ (low-spin) Fe2+ (low-spin) Question : Draw the octahedral crystal field splitting diagram for each metal ion.
1. The formulas and magnetic moments of four octahedral complexes are given below. For each complex, draw the d-orbital splitting diagram and show the locations of the electrons. Calculate the CFSE for each complex in terms of the octahedral splitting energy and the pairing energy. (a) [Fe(H 2 O) 4 (OH) 2] +, magnetic moment = 5.92 B.M.
Feb 28, 2018 · The octahedral splitting energy is the energy difference between the t 2g and e g orbitals. For transition metal complexes called crystal field theory. This diagram shows the field splitting of a metal with ligands in an octahedral configuration. The thick horizontal lines represent atomic orbitals of the metal left and ligands right.
[Cr(ox) 3] 3- has Cr in the +3 oxidation state, which is d 3, and approximately an octahedral crystal field so LFSE = -12Dq. b) hexacyanoferrate(II) ion [Fe(CN) 6] 4- has Fe in the +2 oxidation state, which is d 6, and a strong octahedral crystal field so LFSE = -24Dq + 2P. c) tetrachloro-η 2 -etheneplatinate(II) ion
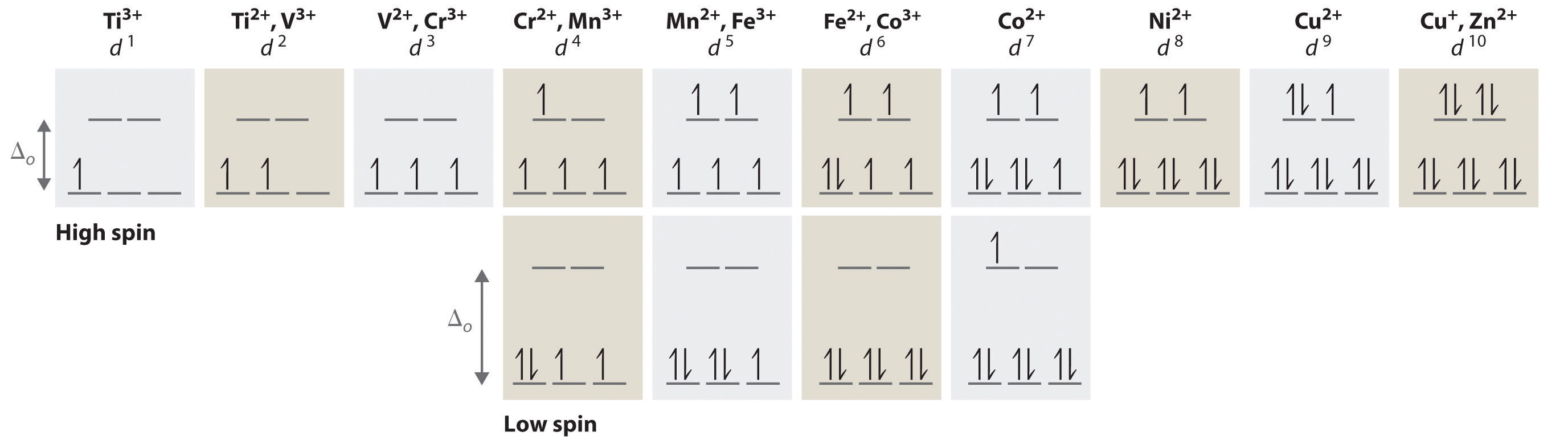
On the left hand side, d 2, d 7 tetrahedral and d 3, d 8 octahedral complexes are covered and on the right hand side d 3, d 8 tetrahedral and d 2 and high spin d 7 octahedral. Again for simplicity, the g subscripts required for the octahedral complexes are not shown. On the left hand side, the first transition corresponds to Δ, the equation to calculate the second contains expressions with ...
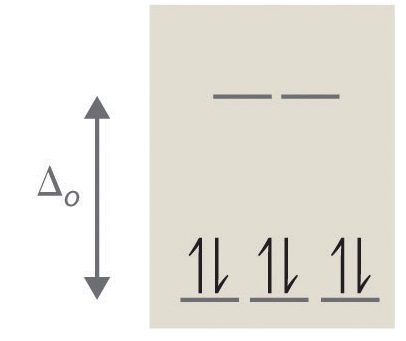
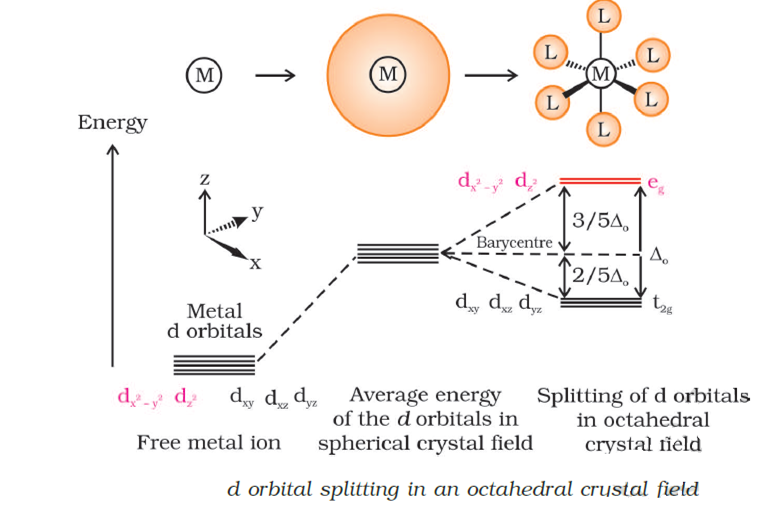
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
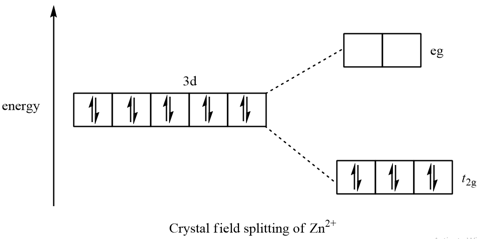
The d orbitals of a metal ion in an octahedral crystal field (surrounded by an octahedral array of ligands) are split into a higher energy eg set and a lower energy t2g set (Figure 1). This is due to electron clouds around each ligand (L) destabilizing those d-orbitals that lie along the X, Y, and Z axes.
That means we can focus on octahedral or tetrahedral complexes (which have very similar crystal-field splitting diagrams). CRYSTAL-FIELD SPLITTING DIAGRAMS All four of these transition metals commonly have coordination numbers of \mathbf(6), however, so let's examine their octahedral complex crystal-field splitting diagrams. HIGH SPIN VS.
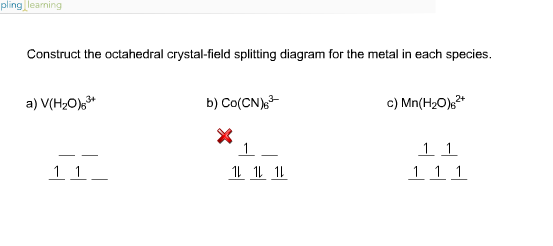
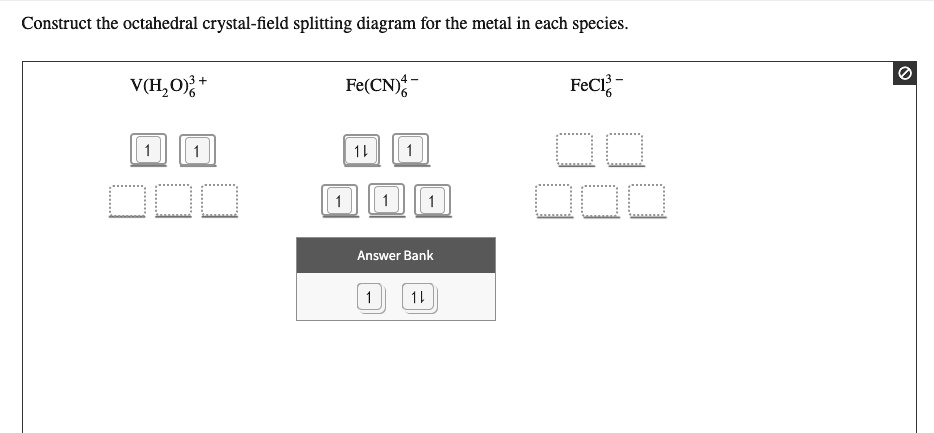
Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
Draw the octahedral crystal field splitting diagram for each metal ion. Zn^2+ Fe^+ (high- and low-spin) The [Mn(NH_3)_6]^2+ ion is paramagnetic with five unpaired electrons. The NH_3 ligand is usually a strong field ligand. Is NH_3 acting as a strong field ligand in this case?
Tetrahedral Crystal Field Splitting! The same considerations of crystal field theory can be applied to ML4 complexes with Td symmetry. •In Td, dxy, dyz, dxz orbitals have t2 symmetry and dx2-y2, dz2 orbitals have e symmetry. ! Relative energies of the two levels are reversed, compared to the octahedral case. " No d orbitals point directly ...
The electron previously filled in d-orbital of the free metal ion can now be considered as distributed between t2g * and e g *. Hence, the crystal field splitting Δ o decreases when ligand to metal bonding takes place. The overall molecular orbital energy level diagram for this type of π-bonding in octahedral complexes can be shown as:
Metal d orbitals in an O h crystal field • If a transition metal ion is placed in a spherical field equivalent to the charges on six ligands, the energies of all five d orbitals would rise together (degenerately) as a result of the repulsions between the negative charges on the ligands and the negative charges of the
assault a criminal planner Complex sound has a slope basically, um, integration off X and also are the in between X and a Y axis. So this and another one lake this. So that means that when a little will approach this complex in that case, it will ah strongly interact with them. Excess square Y square and Exley are because And that means that the store beetles will face the most strong ...
From the oxidation state and the metal\'s group on the periodic table, there are 4 d-electrons present in the metal ion. Knowing that halides are weak-field ligands, the maximum number of unpaired spins (i.e., high spin) is expected in this octahedral complex. That is, in an octahedral-splitting diagram, the three t2g orbitals and two eg ...
Draw figure to show the splitting of d orbitals in an octahedral crystal field. Answer The splitting of the d orbitals in an octahedral field takes palce in such a way that d x 2 y 2 , d z 2 experience a rise in energy and form the eg level, while d xy , d yz and d zx experience a fall in energy and form the t 2g level.
Solved Draw The Octahedral Crystal Field Splitting Diagram Electron Orbitals And Valence Diagram Electron Configuration Electrons Transition Metal. Electron Configurations For The Third And Fourth Periods Video Khan Academy. 1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or ...
The splitting of fivefold degenerate d orbitals of the metal ion into two levels in a tetrahedral crystal field is the representation of two sets of orbitals as T d. The electrons in d x 2 -y 2 and d z 2 orbitals are less repelled by the ligands than the electrons present in d xy , d yz , and d xz orbitals.
Chemistry. Chemistry questions and answers. 1) Draw the octahedral crystal field splitting diagram for each metal ion. a) V3+ b) Co2+ (high-spin) 2)The [CrCl6]3− ion has a maximum in its absorption spectrum at 735 nm. Calculate the crystal field splitting energy (in kJ/mol) for this ion. 3) Which complex ion is diamagnetic? Which complex ion is.
of crystal field splitting energy (10 Dq); and the single absorption band in a UV-vis experiment is exactly what we are looking for. Hence, the energy of the transition 2T2g → 2Eg gives the value of Δ directly. Figure 36. The splitting pattern of free ion term for d1 complexes in the octahedral crystal field.
Draw the octahedral crystal field splitting diagram for each metal ion a cr3 b cu2 c mn3 high and low spin d fe2 low spin
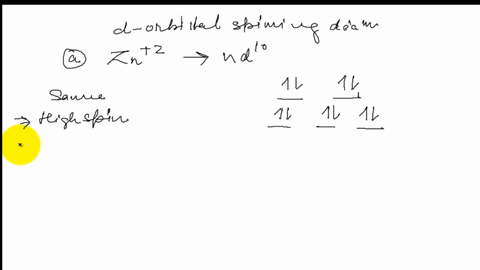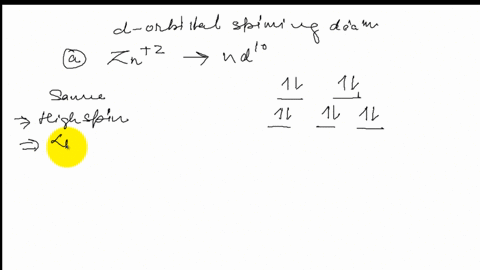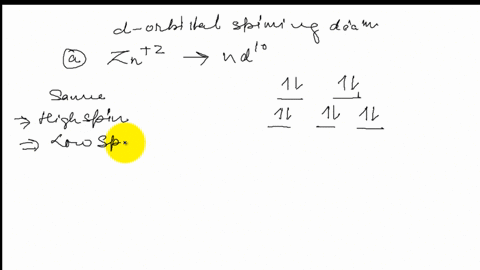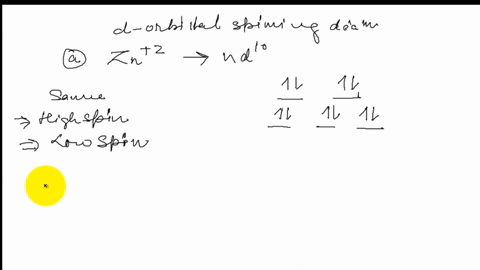








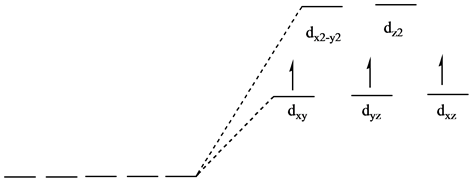





0 Response to "34 draw the octahedral crystal field splitting diagram for each metal ion."
Post a Comment