33 phase diagram questions and answers
Three different kinds of phases are solid, liquid and vapour. 11. What is an equilibrium phase diagram? A phase diagram can be defined as a plot of the composition of phases as a function of temperature in any alloy system under equilibrium condition. 12. What are the advantages of the equilibrium diagrams? 1.
Instructions: Choose an answer and hit 'next'. You will receive your score and answers at the end. question 1 of 3 What is a phase diagram? A graph of the physical state of a substance (solid,...
Materials Science and Engineering: An Introduction answers to Chapter 9 - Phase Diagrams - Questions and Problems - Page 349 9.4 including work step by step written by community members like you. Textbook Authors: Callister, William D.; Rethwisch, David G., ISBN-10: 1118324579, ISBN-13: 978-1-11832-457-8, Publisher: Wiley

Phase diagram questions and answers
Answer. It is useful to accept vapor fractions outside the interval 0 to 1. Just keep in mind that above 1 indicates single-phase vapor, and below zero indicates single-phase liquid. The Wilson ...
Phase diagram questions. 14:440:407 ch9 Question 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the ...
answer sheet. Fe-Fe 3C phase diagram is given on the last page of the exam. _____ Multiple choices (2.5 points each): ____ 1. A phase is defined as a matter with A. distinct composition B. distinct structure C. distinct structure and composition D. all of above ____ 2. See the phase diagram of water. On the liquid/solid boundary line, the ...
Phase diagram questions and answers.
d) Ternary phase diagram. Answer: b Clarification: Binary phase diagrams are based on two component systems. Here, the two components may be mixed in an infinite number of different proportions, which indicates that composition also becomes a variable, along with pressure and temperature. Iron-carbon phase diagram, Pb-Sn diagram are the best ...
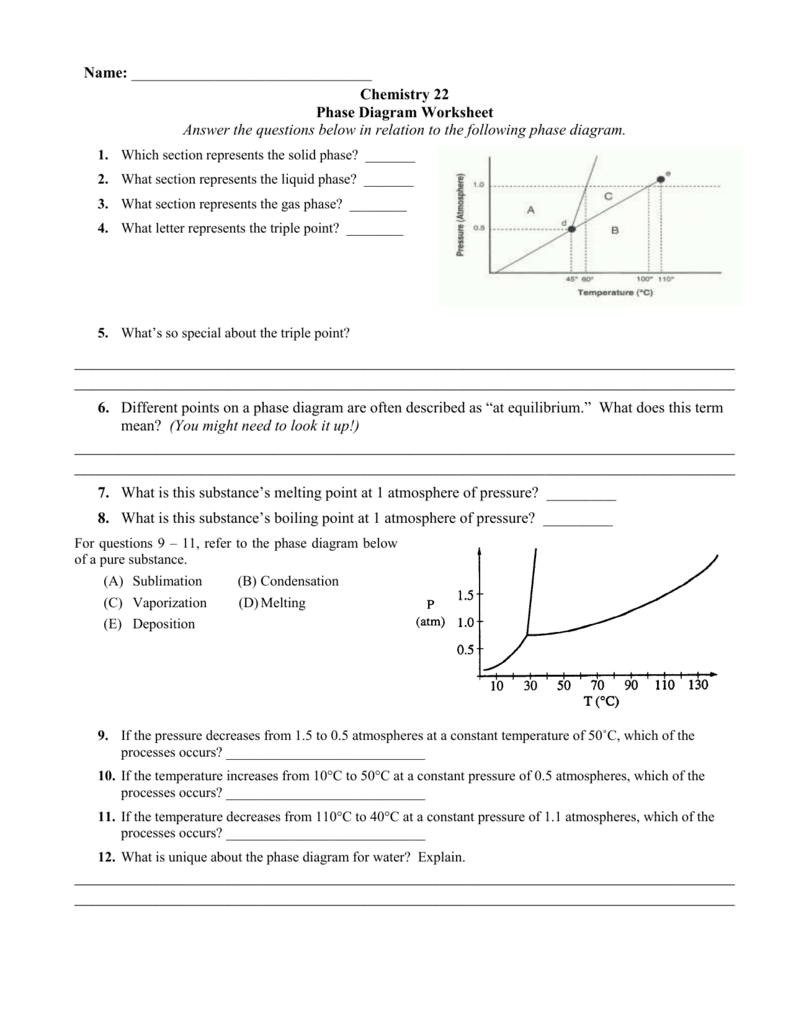
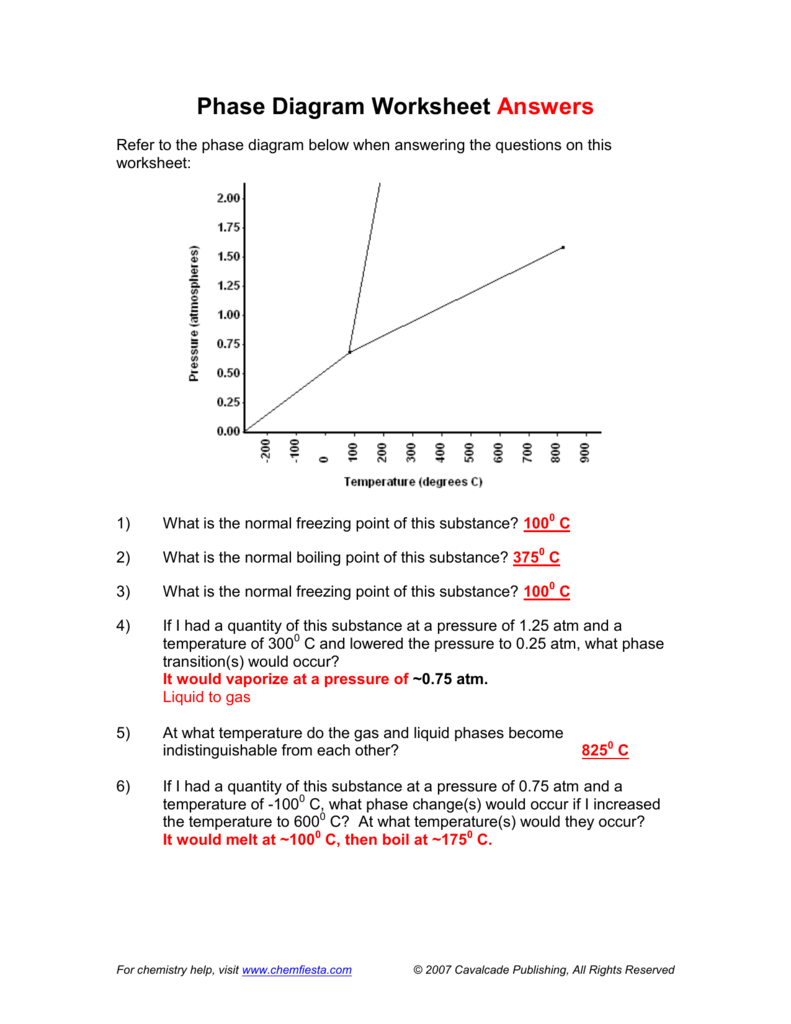
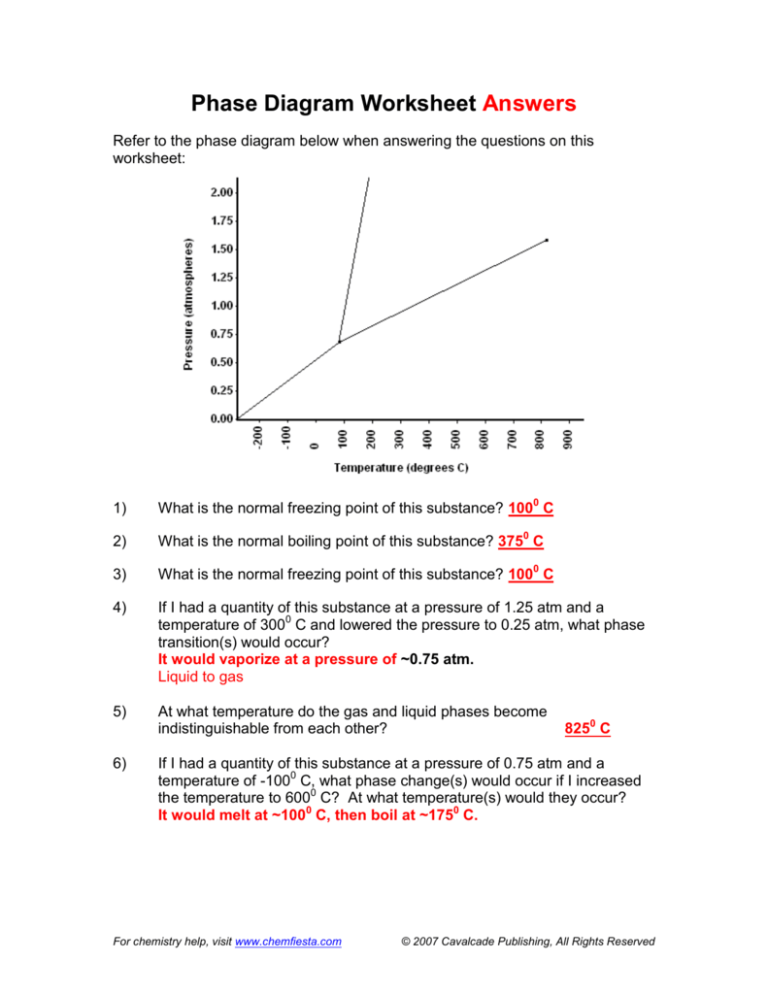
6) If I had a quantity of this substance at a pressure of 0.75 atm and a temperature of -1000 C, what phase change(s) would occur if I increased the temperature to 6000 C? At what temperature(s) would they occur? Phase Diagram Worksheet Answers. Refer to the phase diagram below when answering the questions on this worksheet:
Example Question #1 : Phase Diagrams A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove. The temperature outside is -10 degrees Celsius.
The following questions require some thought and reaching the answer may require you to think beyond the contents of this TLP. Using the following data, calculate the volume fraction of the beta phase and eutectic at the eutectic temperature, for an alloy of composition 75 wt% Ag.
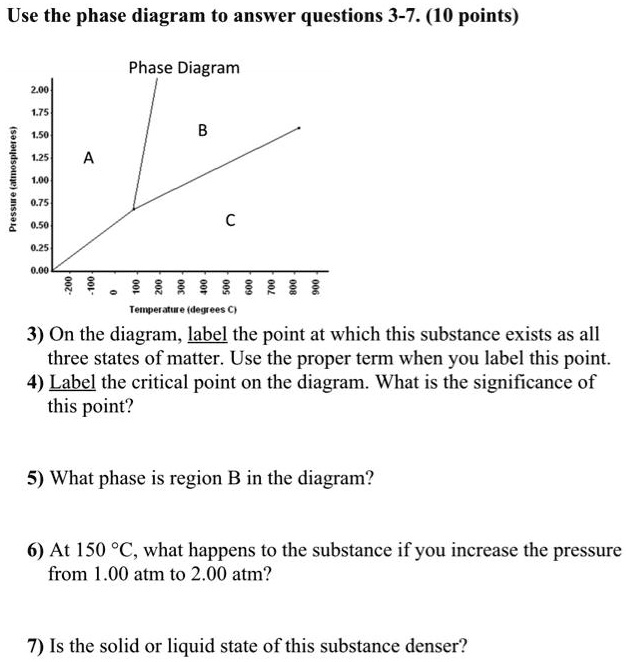
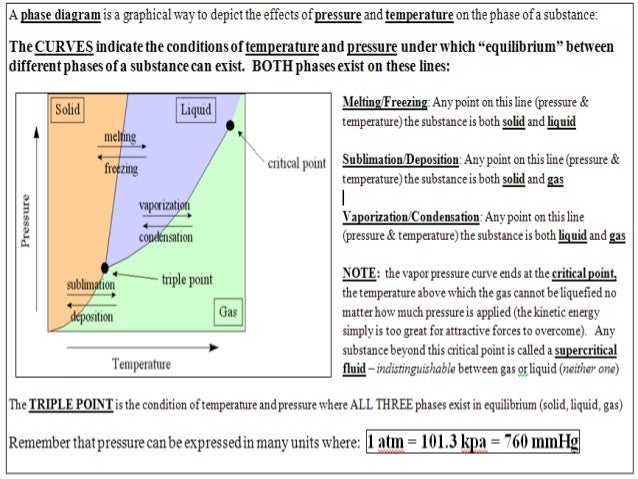
A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance: ... (NOTE: multiple answers needed for this question) 22) If I had a quantity of this substance at a pressure of 2.00 atm and a temperature of -1500 C, what phase change(s) would occur if I decreased the pressure to 0.25 atm? At ...
Importance of Phase Diagrams • There is a strong correlation between microstructure and mechanical properties, and the development of alloy microstructure is related to the characteristics of its phase diagram. • Phase diagrams provide valuable information about melting, casting, crystallization and other phenomena.
Phase equilibria diagrams answer questions about flexibility and constraints of a ceramic or glass component in high-temperature or high-pressure environments. Phase boundaries also assist in the evaluation of the service stability of a ceramic material, both in the long and short time frames. Phase diagrams all the phase diagrams were ...
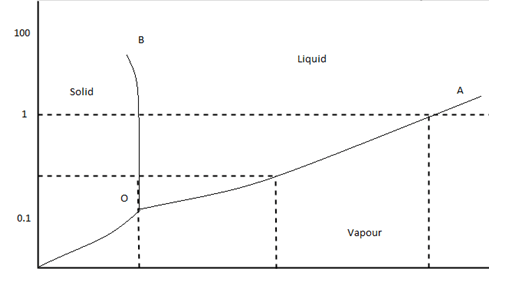
Q. Water exists as a _____________ at 700 mmHg and 50 °C. Q. What phase change (s) may occur at pressures below 4.58 mmHg? Q. The pressure is increased on a sample of water at 0 °C from 0 mmHg to 800 mmHg. In order, what changes occur? Q. Based on this phase diagram, which state is the most dense? Q. Based on its phase diagram, which is the ...
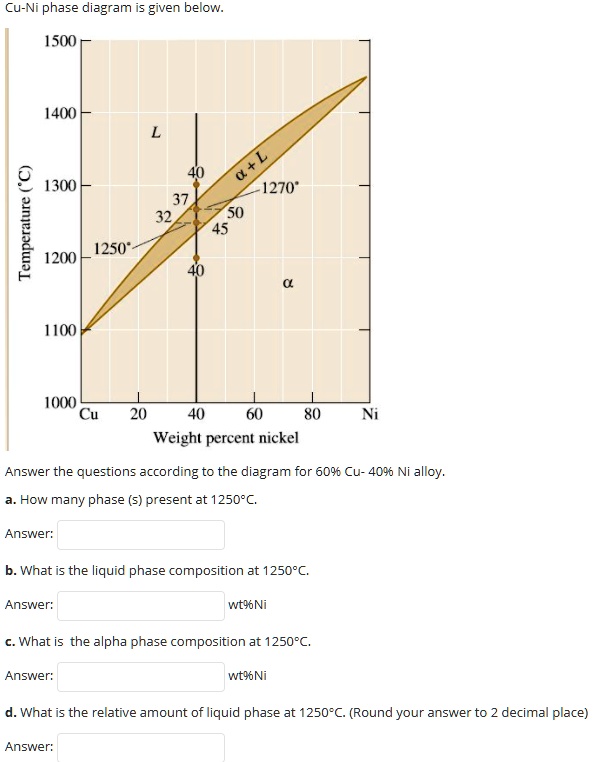
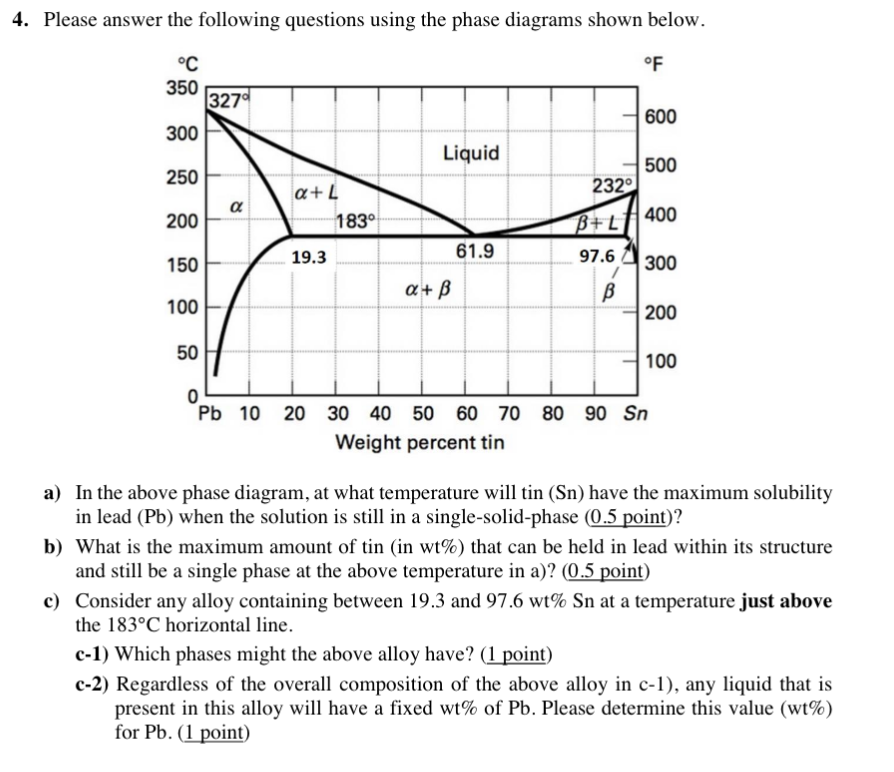
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
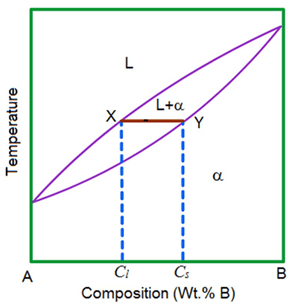
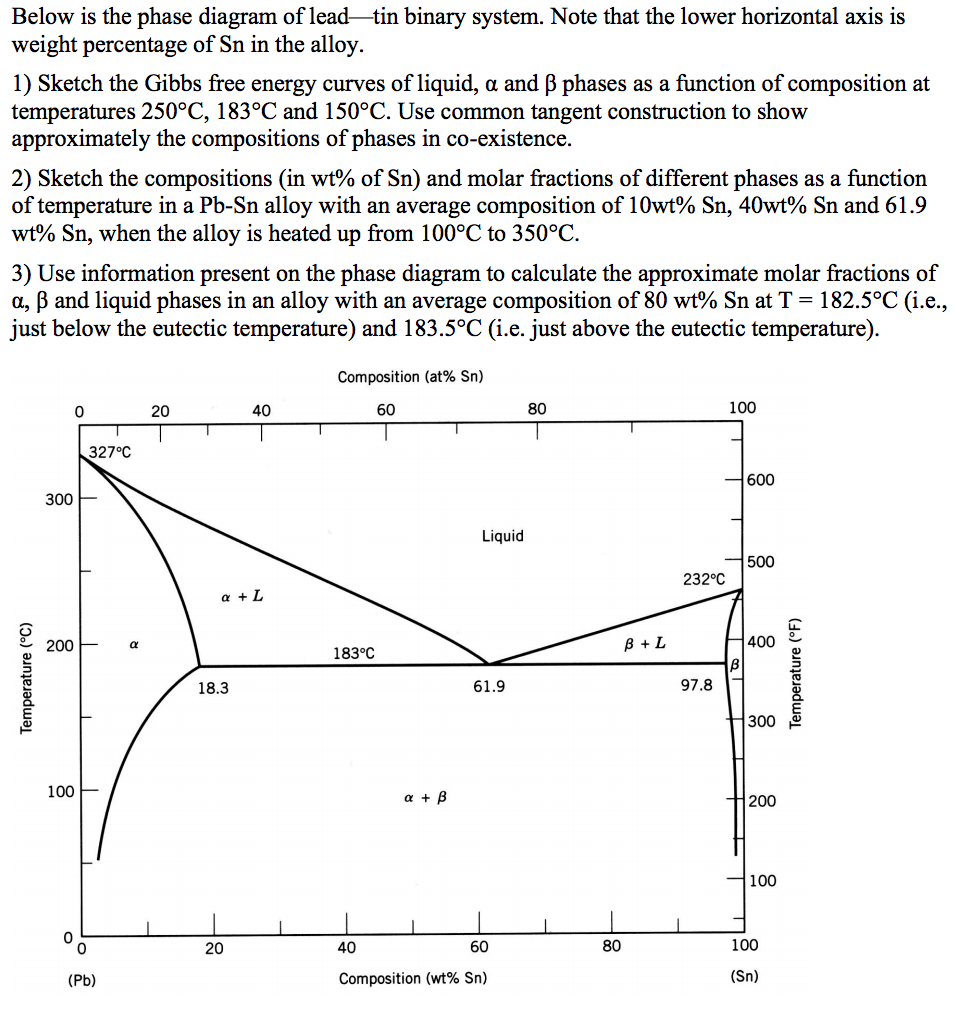
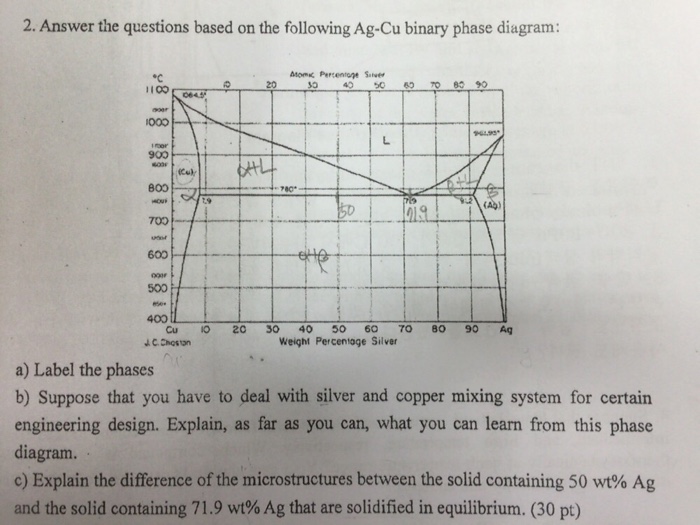
The Cs-K phase diagram is given on the next page. Refer to it in answering the following questions. (a) For a sample of composition 20 at. % Cs and 80 wt. % K held at 10°C, determine (i) the composition of the solid phase present at equilibrium (ii) the composition of the liquid phase present at equilibrium
14 Questions Show answers. Question 1. SURVEY. 30 seconds. Q. What state of matter is Y? answer choices. solid. liquid.
You'll be practicing these skills as you answer the questions on this quiz: Reading comprehension - ensure that you draw the most important information from the related lesson on the phase diagram ...
Explore the latest questions and answers in Phase Diagrams, and find Phase Diagrams experts. Questions (157) Publications (89,833) Questions related to Phase Diagrams. 1. 2. Mustafa Tuncer.
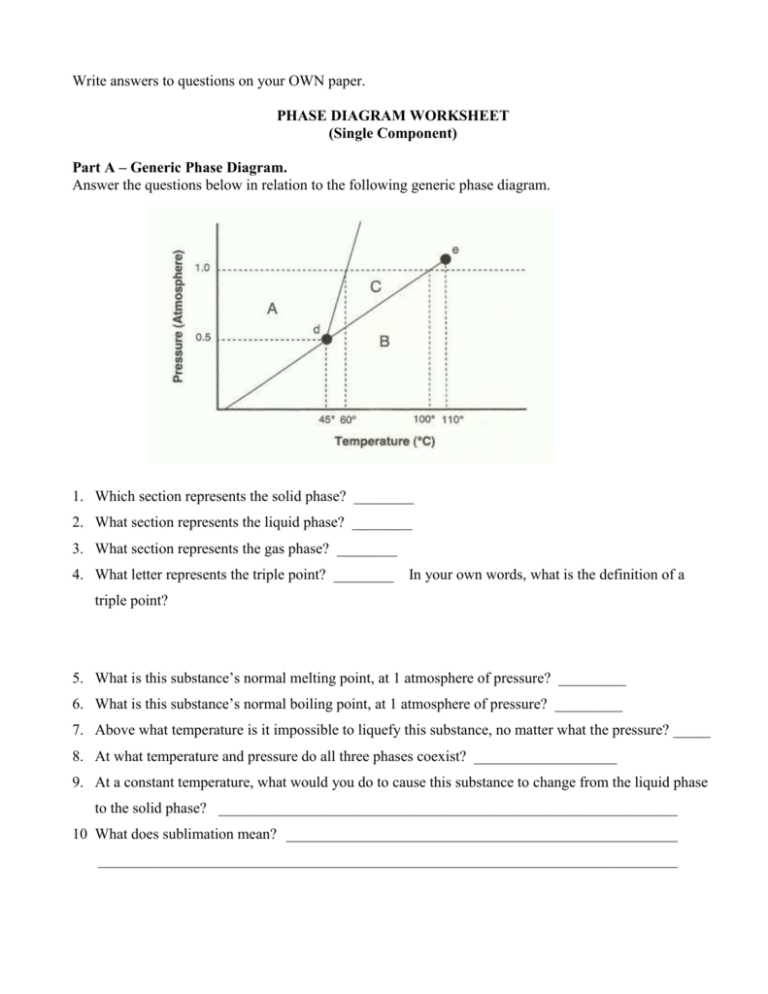
Phase Diagram Worksheet Answers Refer to the phase diagram below when answering the questions on this worksheet: 1.75 1.50 1.25 0.75 0.50 0.25 0.00 Temperature {degrees C) 2) 3) 4) 6) Label the following on the phase diagram above: Solid phase, liquid phase, gas phase, triple point, critical point.
Answers to Chemistry Problems Answers to Chemistry Problems; Chemistry Quiz Online Quizzes for CliffsNotes Chemistry QuickReview, 2nd Edition; Quiz: Phase Diagrams Previous Phase Diagrams. Next Heat Capacities and Transformations. Discovery and Similarity Quiz: Discovery and Similarity Atomic Masses ...
are a single phase. Questions ... H3.2.5 Answers to questions: section H3.2 H3.2.1 W Cu ¼ 70%,X Cu ¼ 71%,X ... Definition An equilibrium diagram (or phase diagram) is a diagram with T and X B (or W B) as axes, showing the equilibrium constitution. 2064 Teachyourselfphasediagrams
Use the graph to answer the following questions. A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance. Heating Curves Biology Worksheet Quizzes And Answers Chemistry 175 150 125 075 050 025 000 Temperature degrees C 2 3 4 6 Label the following on […]
Answer: c Explanation: A unary phase diagram has a single component thus, at triple point, the number of degrees of freedom, according to Gibbs phase rule F = 1 - 3 + 2 = 0.
Material Science/ Phase Diagrams Multiple Choice Questions Answers: 1. d 2. c 3. a 4. c 5. a 6. c 7. a 8. c 9. b 10. a 11. b 12. b
Cu-Ni phase diagram wt% Ni 20 1200 1300 T(°C) L (liquid) α (solid) L + α l i q u i d u s s o l i d u s 30 40 50 TA A D TD TB B tie line L + α 3235 43 CLCo Cα R S Information we can extract from the diagram: ¾the phases present; ¾composition of the phases ¾percentage of fraction of the phases Composition at T B: • Liquid phase (L) of ...
Materials Science and Engineering: An Introduction answers to Chapter 9 - Phase Diagrams - Questions and Problems - Page 352 9.51 including work step by step written by community members like you. Textbook Authors: Callister, William D.; Rethwisch, David G., ISBN-10: 1118324579, ISBN-13: 978-1-11832-457-8, Publisher: Wiley
a) Melting temperatures of various phases. b) Temperature range for solidification. c) Equilibrium solid solubility. d) Purity of materials. View Answer. Answer: d. Explanation: A phase diagram is a graphical representation of the phases present in the system of materials at various temperatures, pressures, and compositions.
Phase Transformation Multiple Choice Questions & Answers (MCQs) on "Thermodynamics and Phase Diagrams - Equilibrium". 1. The relative stability of a system for transformations that occur at constant temperature and pressure is determined by its _____
















0 Response to "33 phase diagram questions and answers"
Post a Comment