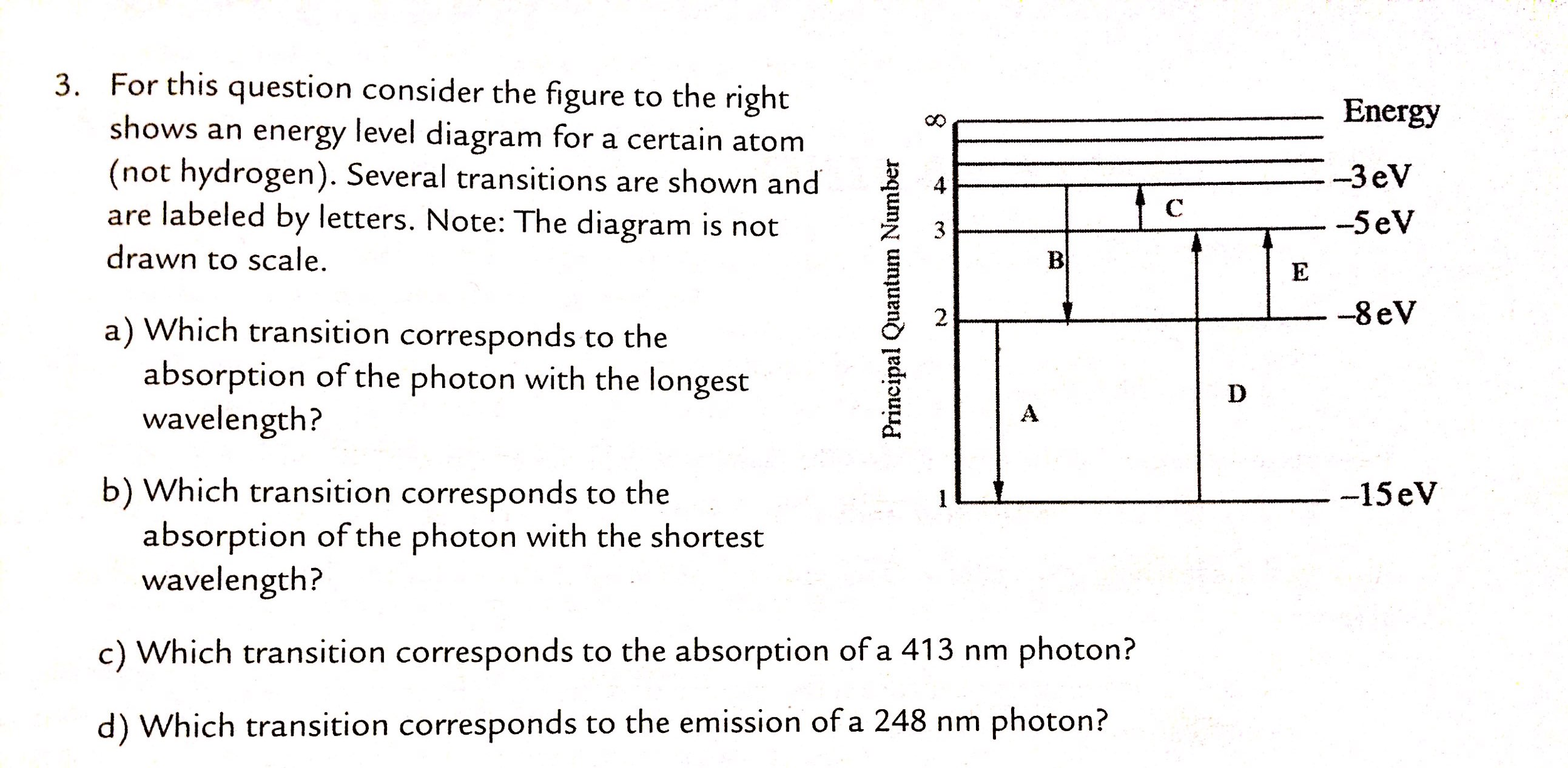
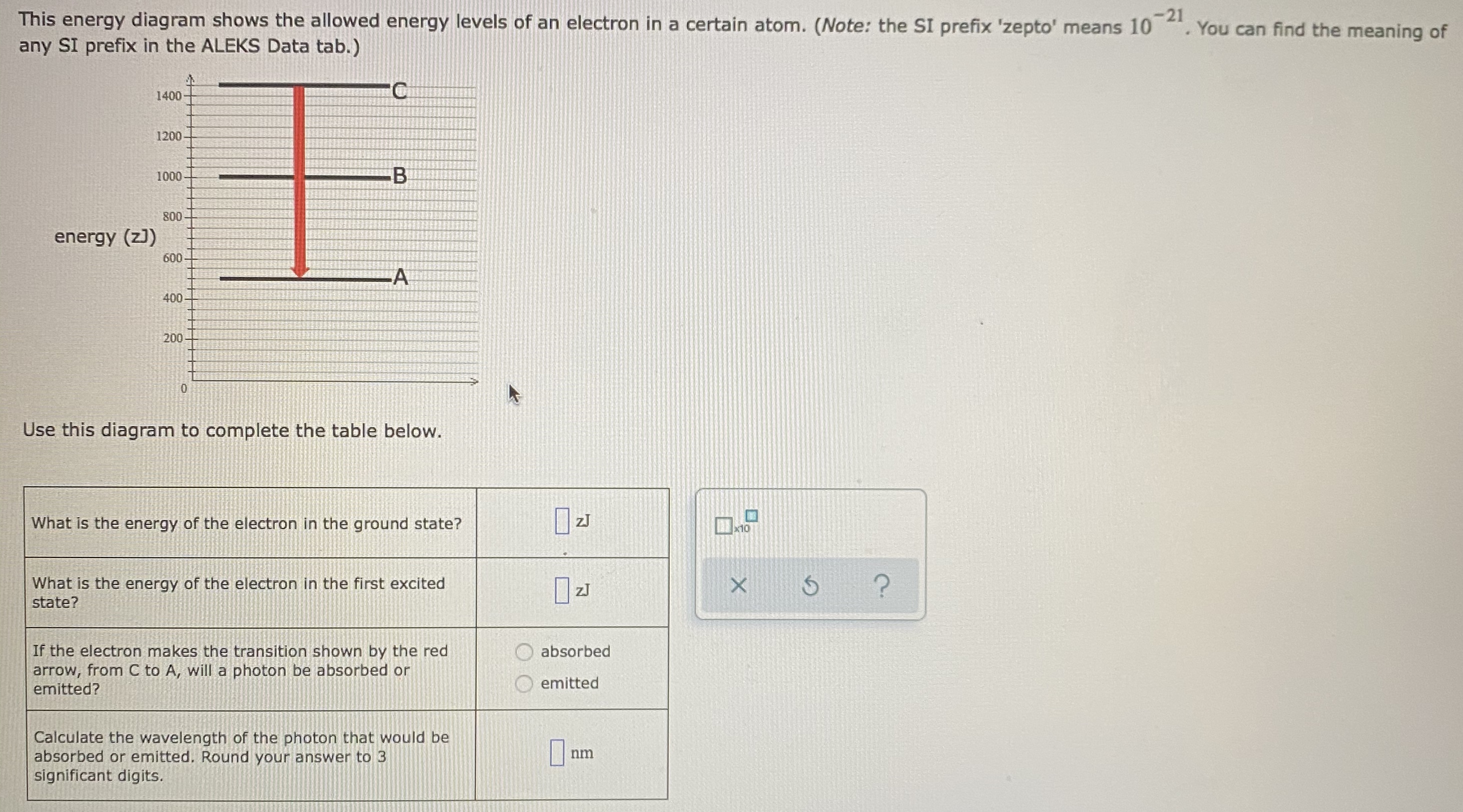
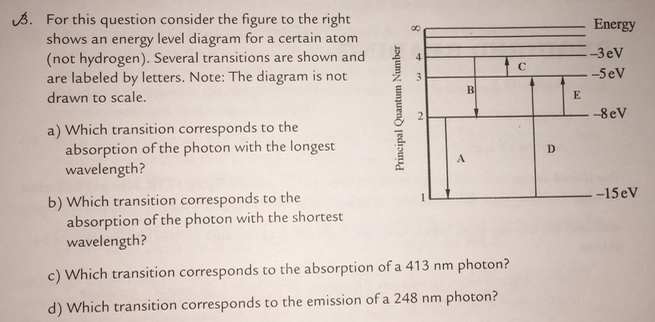
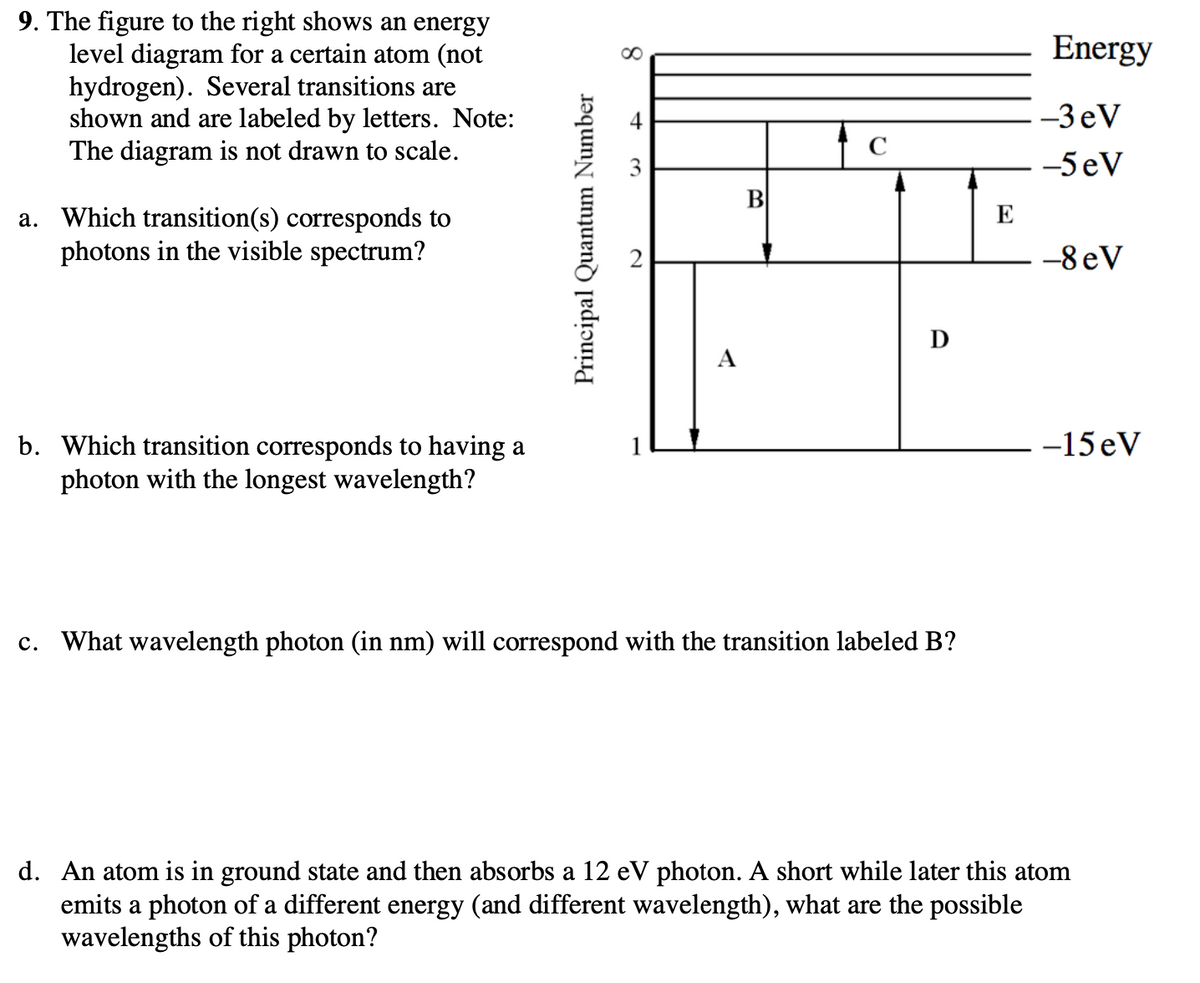
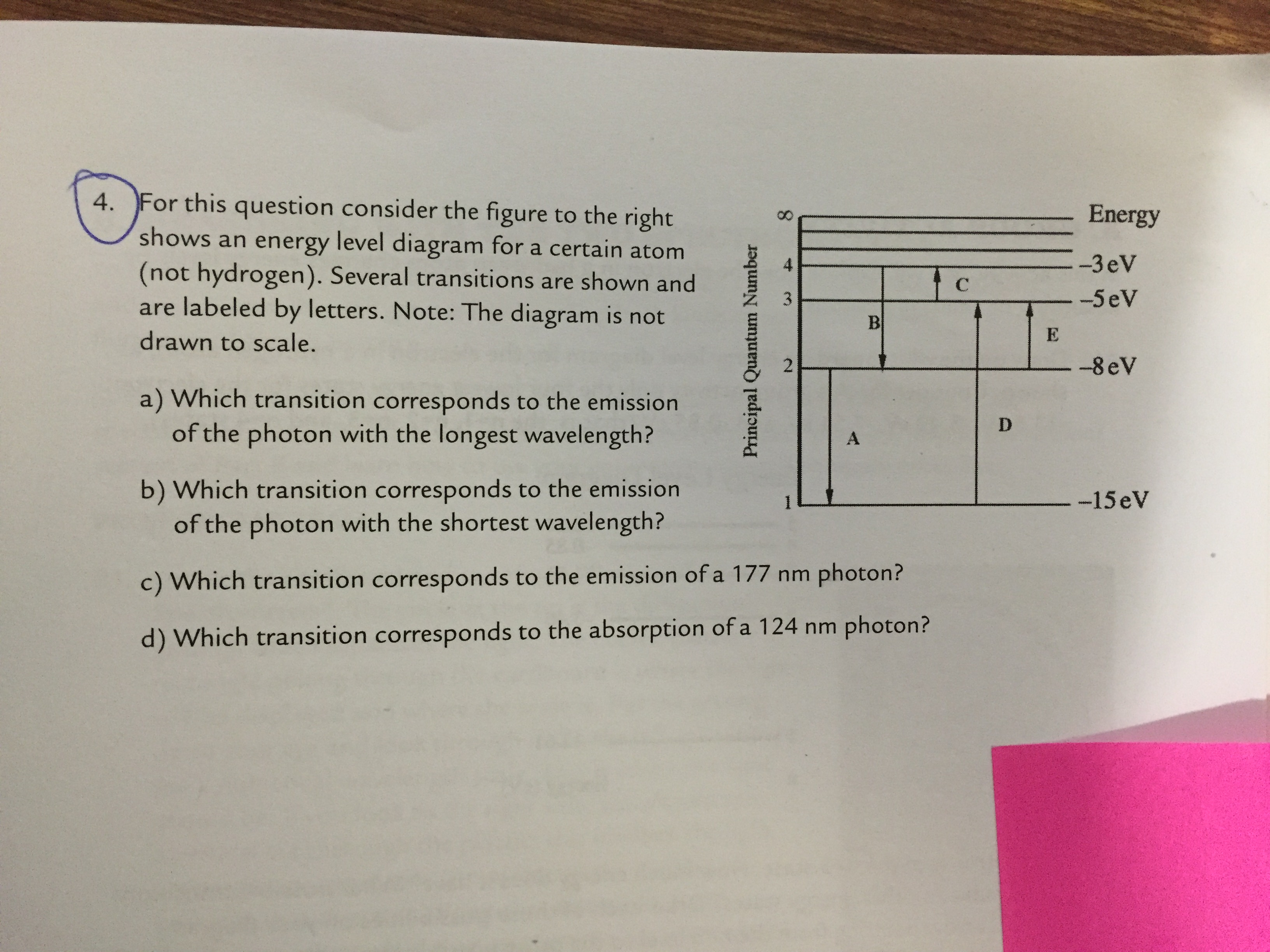
33 for this question consider the figure to the right shows an energy level diagram for a certain atom
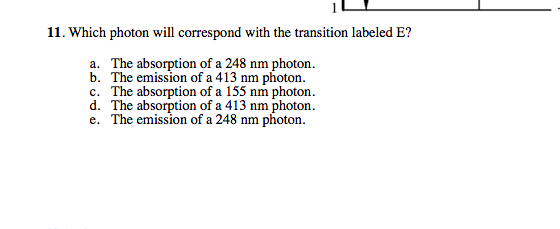
Figure 2 Refer to Figure 2 when answering questions #10 and #11. 10. In the two-level atom shown in Figure 2, which electron transition is associated with the emission of light? ANSWER. The transition labeled "b". 11. If the "c" transition marked in the three-level atom is associated with the absorption or
The electrons in an atom move around the nucleus in regions known as electron shells. Each electron shell can only contain a certain number of electrons. Electrons are placed in the lower energy level first and when this is completely filled, we move on to the second energy level and so on. The way the electrons are arranged in an atom is called the atom's electronic structure or electronic ...
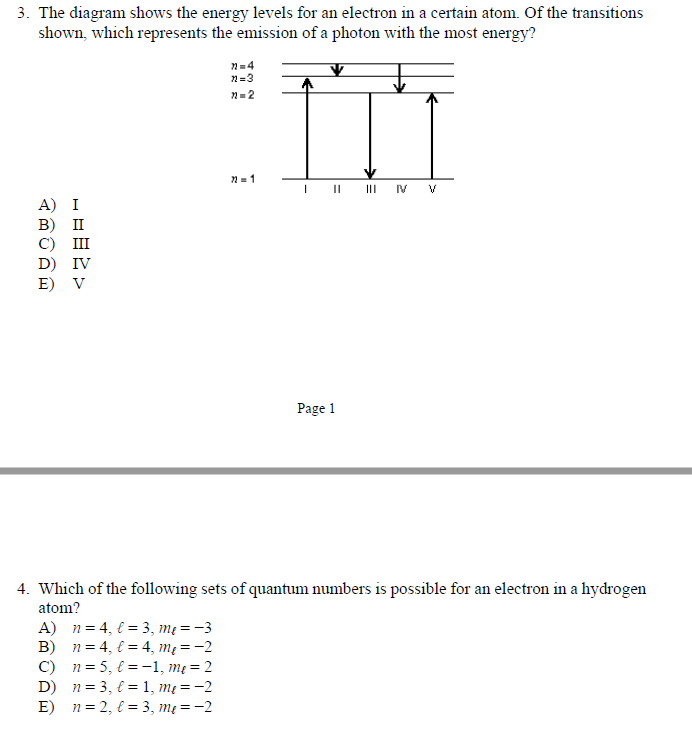
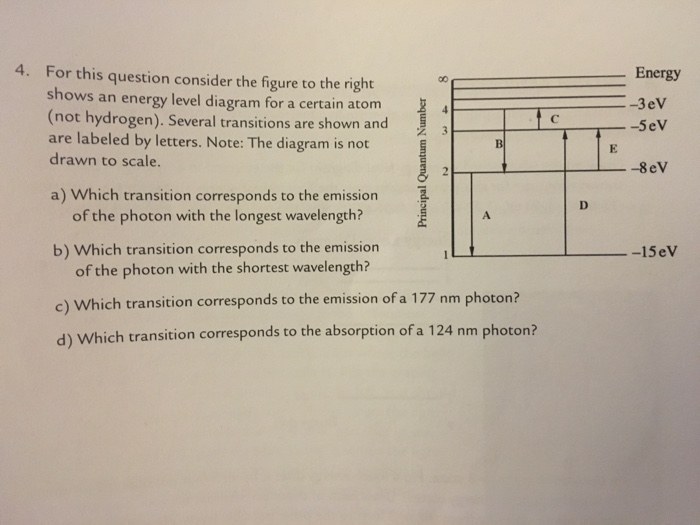
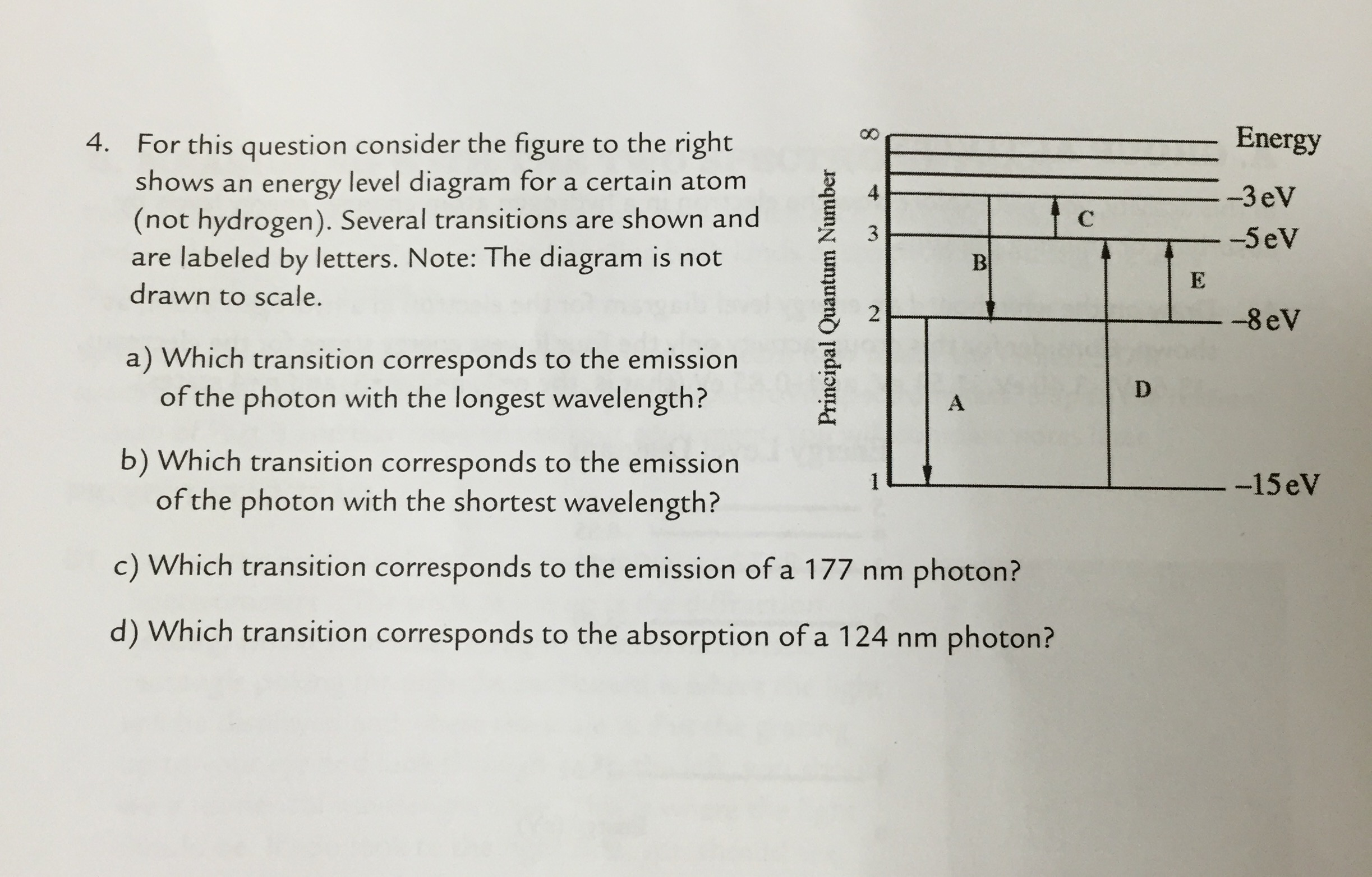
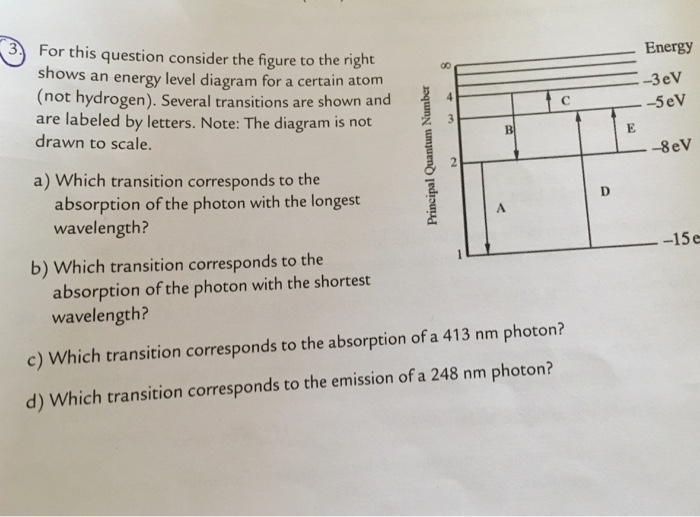
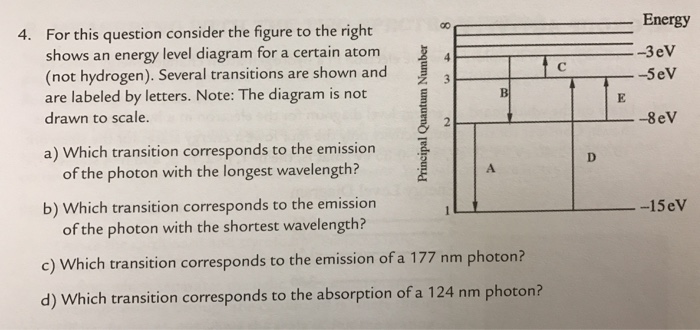
For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labelled by letters. Note: The diagram is not drawn to scale.
For this question consider the figure to the right shows an energy level diagram for a certain atom
atom, (ii) from the carbon atom to a point midway between the fluorine atoms, (iii) from the carbon atom to a point mid-way between the hydrogen atoms, or (iv) From the carbon atom toward a hydrogen atom? [Sections 9.2 and 9.3] 9.5 The following plot shows the potential energy of two Cl atoms as a function of the distance between them.
Figure 3-2: A potential and total energy diagram.? Why should we draw the total energy as a flat line, while the potential energy changes in situations where we ignore friction? Consider a car which has the total energy indicated in Figure 3-2. It is approaching a set of magnets from the right that have the potential energy represented by the solid
For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. a.) Which transition corresponds to the absorption of the photon with the longest wavelength? b.)
For this question consider the figure to the right shows an energy level diagram for a certain atom.
Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows: s ...
Oct 06, 2020 · For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength?
The excited state electron configuration of an atom indicates the promotion of a valence electron to a higher energy state. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. Example The ground state electron configuration of sodium is "1s"^2"2s"^2"2p"^6"3s"^1. In its excited state, the valence electron in the "3s ...
(Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.) 1._____contains all the electrons with similar energy. 2._____ is the region around the nucleus where electrons of a certain energy are most likely to be found.
enerw level 5 to energy level 2. Refer to Models 1 and 2 for the following questions. a. Label the picture with "n=5 to n=2" and list the corresponding color of light emitted. b. This electron transition (absorbs release energy. c. This electron moves from a (low [higher energy state to a lowe igher) energy state. d.
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...
After the second energy level has 8 electrons (neon), the next electrons go into the third energy level. After the third energy level has 8 electrons (argon), the next 2 electrons go into the fourth energy level. Note: The third energy level can actually hold up to 18 electrons, so it is not really filled when it has 8 electrons in it. But when ...
For certain atom,there are energy levels A,B,C corresponding to energy values E A < E B < E C .Choose the correct option if λ 1 , λ 2 , λ 3 are the wavelengths of radiations corresponding to the transition from C to B,B to A and C to A respectively.
Solution for Calculate the Energy! Student Worksheet Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on.Remember that there is a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is ...
We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
The equation also shows us that as the electron's energy increases (as n increases), the electron is found at greater distances from the nucleus. This is implied by the inverse dependence on r in the Coulomb potential, since, as the electron moves away from the nucleus, the electrostatic attraction between it and the nucleus decreases, and it is held less tightly in the atom.
For this question consider the Figure to the right shows an energy level diagram for a certain atom Published on 15 days ago 1 min read Category Chegg Answers , PHYSICS
Pls help! For this question consider the Figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition...
Figure 4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
The diagram shows Niels Bohr's model of an atom. What happens when the electron moves from the first energy level to the second energy level? Energy is absorbed, and an emission line is produced. Energy is released, and an emission line is produced.[wrong] Energy is absorbed by the atom. Energy is lost from the atom.
Energy levels are nothing but the fixed distances of electrons from the nucleus of an atom. The energy levels are also called electron shells. An electron can move in one energy level or to another energy level, but it can not stay in between two energy levels. Image will be uploaded soon. The figure shows the energy levels of an atom.
4.3.1 The Hydrogen atom In the previous chapter we studied stationary problems in which the system is best described as a (time-independent) wave, "scattering" and "tunneling" (that is, showing variation on its intensity) because of obstacles given by changes
Physics questions and answers. For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Which transition corresponds to the emission of the photon with the longest wavelength?
How many p electrons are there in an atom of rubidium? (a) 12 (b) 18 (c) 24 (d) 9 (e) 6 20. A neutral atom of an element has 2 electrons in the first energy level, 8 in the second energy level and 8 in the third energy level. This information does not necessarily tell us: (a) the atomic number of the element.
For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (A) Which transition corresponds to the emission of the photon with the longest wavelength?
Figure 2 shows the oscillation of the damped system after the wheel goes over a bump in the road. Figure 2 (i) Describe the energy changes that occur during the time covered by Figure 2, making reference to the types of energy involved at P, Q and R.
item select "Eigenvalue" Æ "Level Diagram". You should see an energy level diagram with two energy levels above and below a dotted line (similar to the one in Figure 5). The dotted line corresponds to α, the energy of the 2py atomic orbitals. The lower level is labeled with an energy of 1.00 and this corresponds to α + 1.00β.
Question: For this question consider the figure to the right shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength?
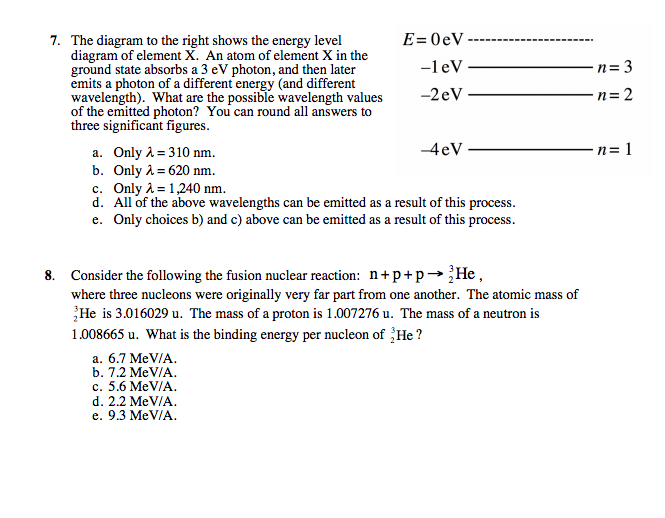
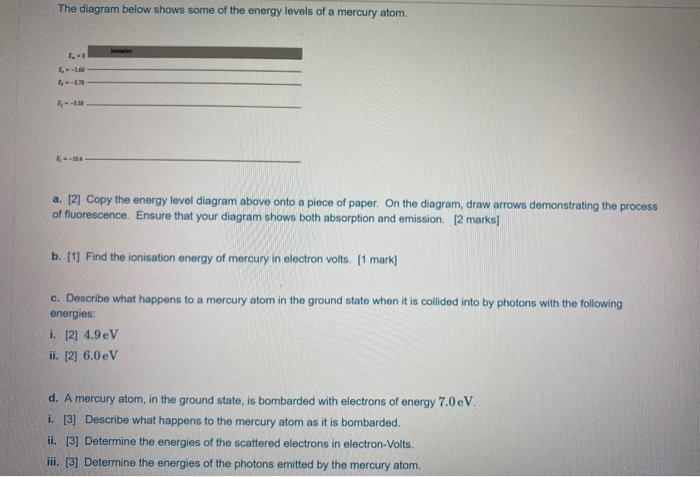
The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
Two rockets, A and B, approach each other as shown in the figure. A travels to the right at 0.7c while B travels to the left at 0.8c. Both speed measurements are made relative to an inertial observer. Both rockets have a supply of unstable mesons with a mean proper lifetime of 2.6 × 10-8 s.
Problem: For this question consider the figure that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and are labeled by letters. Note: The diagram is not drawn to scale. Which transition corresponds to the emission of the photon with the longest wavelength?
Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.










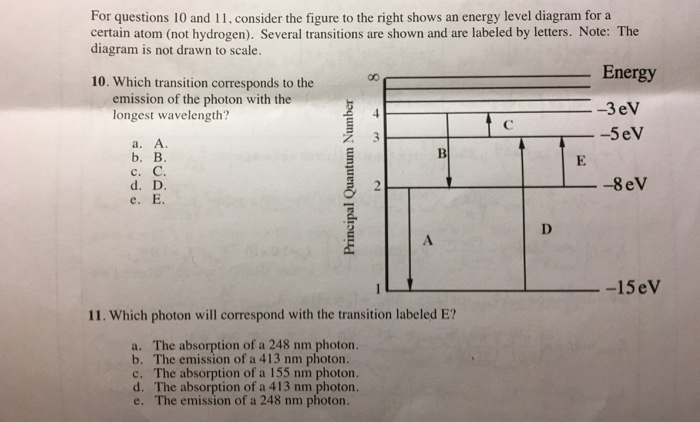

0 Response to "33 for this question consider the figure to the right shows an energy level diagram for a certain atom"
Post a Comment