39 lewis diagram for ccl4
17.06.2021 · Reduced infiltration of anti-tumor lymphocytes remains a major cause of tumor immune evasion and is correlated with poor cancer survival. Here, … 1:50Hey Guys,In this video we are going to learn about the Lewis structure of CCl4. It is a chemical formula for ...8 Feb 2021 · Uploaded by Geometry of Molecules
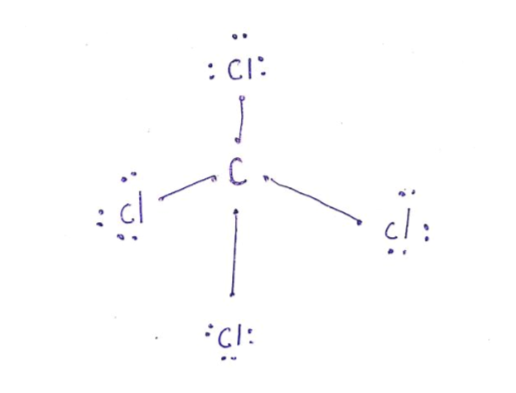
CCl4 lewis’s structure is made up of one carbon atom that is situated at the middle position and four chlorine atoms that are at the surrounding position. The total lone pair present in the CCl4 lewis dot structure is 12. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram.

Lewis diagram for ccl4
1:47CCl4 Lewis Structure How to Draw the Dot Structure for CCl4 Carbon Tetachloride. 737 views737 views. Mar ...7 Mar 2019 · Uploaded by Alyssa Ellingson Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable. For the Lewis structure of CCl4 first, let’s calculate the total valence electrons. The Lewis structures in Figure 1 indicate that the ozone molecule has two equivalent resonance structures, which means the electrons are delocalized. From the Lewis structure, we see that the bond order for O2 is 2 (a double bond), whereas the bond order for O3 is 1.5 (one and a half bonds).
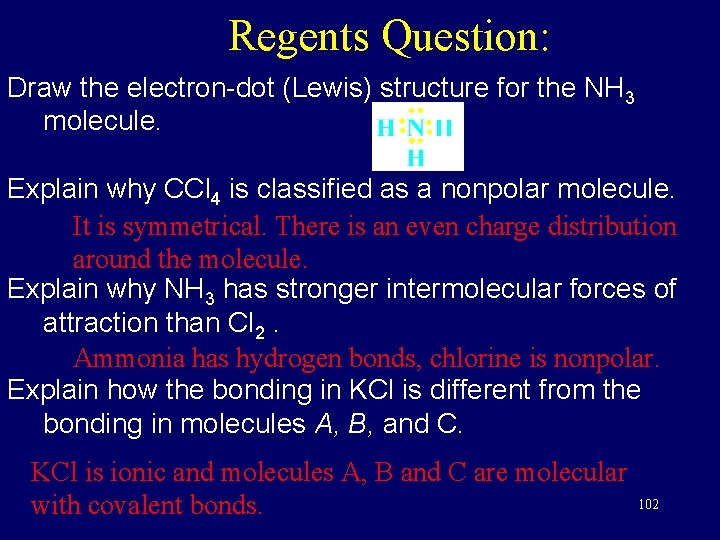
Lewis diagram for ccl4. A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. A regular atom of carbon has 4 lone electrons in its outer shell. A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc... A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds.Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.. Carbon tetrachloride (CCl 4) is a covalently bonded compound composed of a central carbon surrounded by 4 chlorine atoms in a tetrahedral ... Draw the Lewis structure for CO32- including any valid resonance structures. Which of the following statements is TRUE . The CO32- ion contains two C-O single bonds and one C=O double bond. How many of the following elements can form compounds with an expanded octet? P Kr Xe B. 3. Place the following in order of decreasing magnitude of lattice energy. NaF RbBr KCl. NaF > KCl > RbBr. Using ...
Cyanide (CN-) lewis structure, Molecular orbital diagram, bond order, formal charge, and its hybridization. Home > Chemistry Article > CN- lewis structure and its molecular orbital diagram. Cyanide is a chemical compound with the chemical formula CN‾ ion. It is a member of the Cyano group. It is lethal for every living thing and smells like bitter almonds. In this article, we will study the ... The Lewis diagram for CCI4 is: The electron-pair geometry around the C atom in CCL4 is There are lone pair(s) around the central atom, so the geometry of CCl4 is H-C-H B. The Lewis diagram for CH is: The electron-pair geometry around the C atom in CH is There arelone pair(s) around the central atom, so the geometry of CH is 1:226.03 Draw the Lewis structure for CCL4. Watch later. Share. Copy link. Info. Shopping. Tap to unmute. If ...24 May 2016 · Uploaded by Allison Soult This problem has been solved! Draw the Lewis structure of CCl4. Include all the lone pairs. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Draw the Lewis structure of CC14.
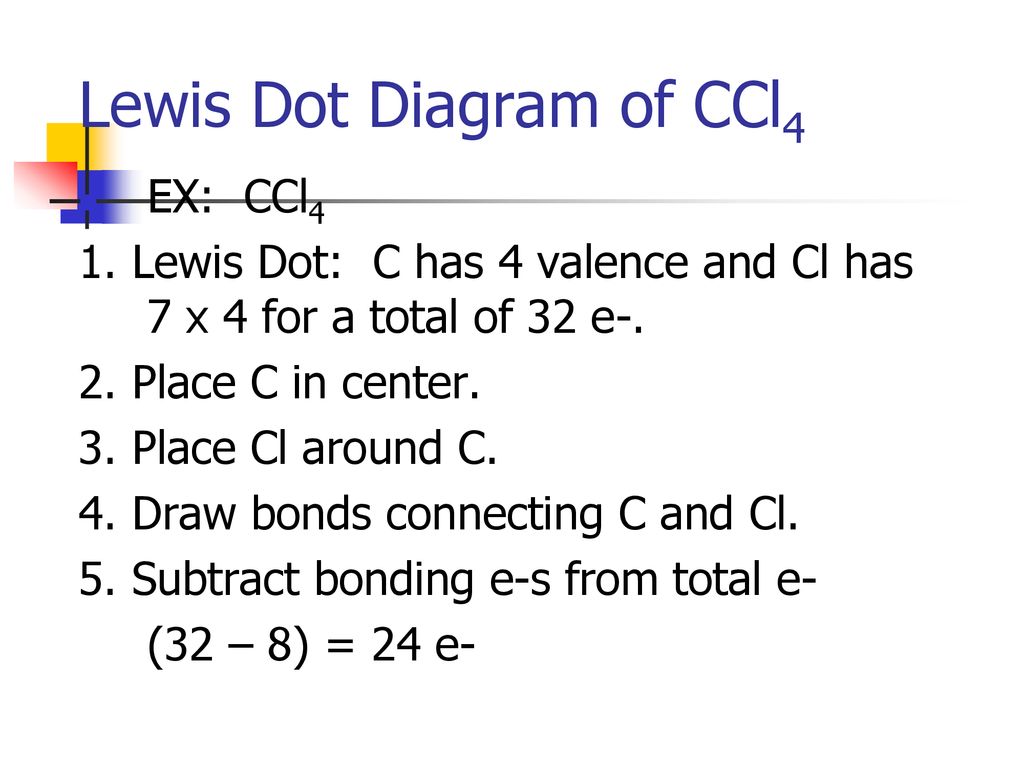
What Is The Lewis Structure For Ccl4. ccl4 is also called Carbon Tetachloride. We called it corbon Tetachloride because C mean corbon and cl mean chloride and 4 call as teta in chemistry so it is called corbon Tetachloride.. The levis structure is diagram That Represent That Configuration Of Electron Of Covalently Bonded Compounds. PowerPoint Presentation Draw Lewis Dot Structures Draw the Lewis Dot Diagram for polyatomic ions Draw Polyatomics Types of Covalent Bonds Types of Covalent Bonds Place these molecules in order of increasing bond polarity which is least and which is most? non-polar MOLECULES Polar molecules (a.k.a. Dipoles) PowerPoint Presentation Space filling model “Electron-Cloud” model Water is ... Lewis structure: AB 3 = trigonal planar, 3-D O3 Molecular Geometry. Therefore this molecule is polar. Directions: (1) Draw the Lewis Structure. It is an AX4 system: tetrahedral. 2021-02-27. This activity draws some extra concepts and mathematical skills into the discussion of molecular shape. It is a clear, colorless gas. PCl 4 + g. Important! If you have come straight to this page via a ... As an example, let's use carbon tetrachloride, CCl4. The single carbon atom contains four valence electrons, and each of the four chlorine atoms contains seven ...
The lewis dot structure of OF2 is very easy to draw if you follow the simple approach of drawing the lewis diagram. There is a total of 16 lone pair electrons and 4 bonded pair electrons present in the OF2 lewis structure. Steps to follow for drawing the OF2/F2O lewis structure . 1. Count total valence electron present in OF2. In the first step, you need to find how many valence electrons are ...
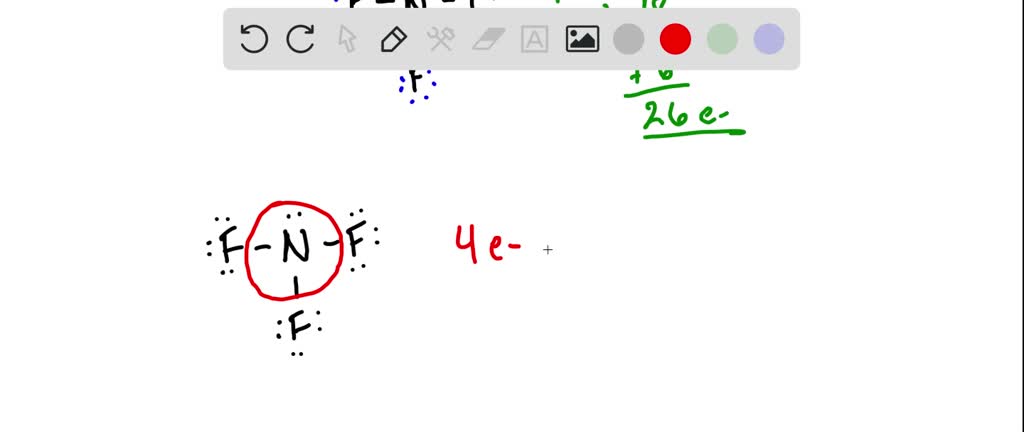
Solved Please Draw The Lewis Structure For Ccl4 Please Show Work And Any Formal Charges What Is The Electron Pair Geometry About The Central N What Are The Bond Angles About The Centr4al
2:05How to Draw a Lewis Structure for CCl4? Lewis Structure: ...15 Jun 2019 · Uploaded by Dr. Masi
1:16I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over ...1 Oct 2011 · Uploaded by kentchemistry.com
CCl4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Carbon Tetrachloride is a chemical that is produced and is not available naturally. In its natural state, it is a colorless liquid chemical with a little sweet smell like ether.

Give The Number Of Valence Electrons And Draw The Lewis Structure For The Following Species A Ccl4 B Pbr3 C H2o D C2hf E Sio32 Study Com
Estructura de Lewis del CCl4 (Tetracloruro de Carbono) Si queremos realizar la estructura de Lewis del CCl 4 (Tetracloruro de Carbono) seguiremos lo siguientes pasos. Comience por dibujar la estructura general de la molécula del CCl 4 uniendo los átomos de cloro con el carbono con enlaces simples: Ahora contaremos los electrones de valencia ...
The Lewis structures in Figure 1 indicate that the ozone molecule has two equivalent resonance structures, which means the electrons are delocalized. From the Lewis structure, we see that the bond order for O2 is 2 (a double bond), whereas the bond order for O3 is 1.5 (one and a half bonds).
Sticks or straight lines represent the bonds. While the dots represent the non-bonding electrons. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable. For the Lewis structure of CCl4 first, let’s calculate the total valence electrons.

48 Problem 4 If Atomic Mass Of Carbon Was Taken As 100 U Then What Would Be The Value Of Avogadro S Number
1:47CCl4 Lewis Structure How to Draw the Dot Structure for CCl4 Carbon Tetachloride. 737 views737 views. Mar ...7 Mar 2019 · Uploaded by Alyssa Ellingson
Draw The Lewis Dot Structure Of A Al2o3 B Mg3n2 C Ccl4 D Ncl3 E Ncl3 Sarthaks Econnect Largest Online Education Community
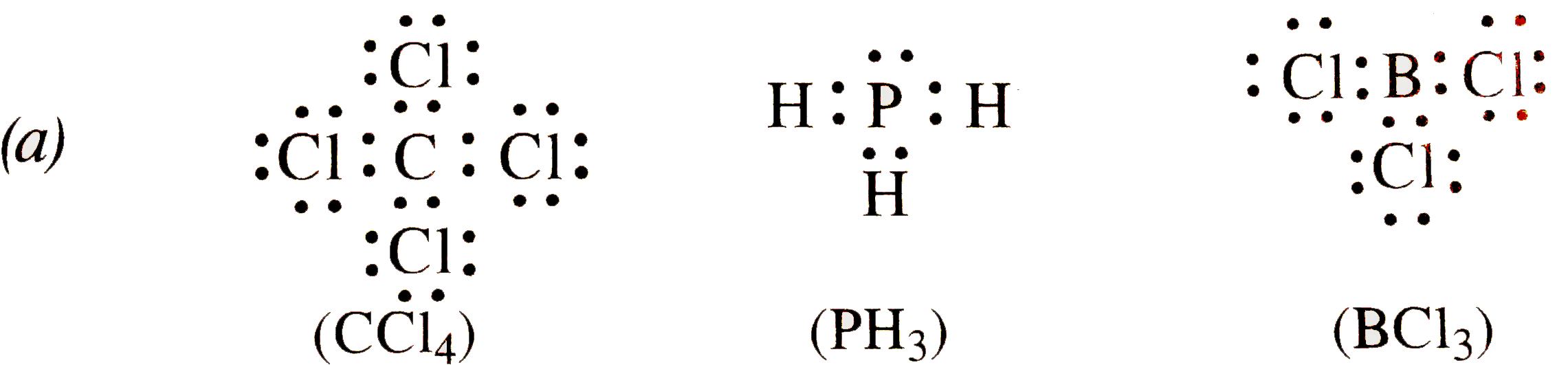
Write The Lewis Dot Structures Of A C Cl 4 B Ph 3 C Bcl 3 Is The Octet Roule Obeyed In These Structures
Draw The Electron Dot Structure Of O2 Nh3 And Ccl4 Science Carbon And Its Compounds 10050281 Meritnation Com




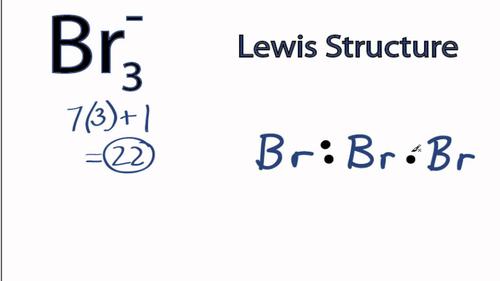

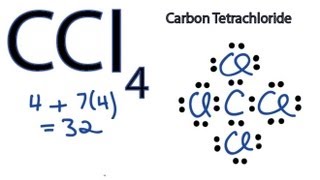



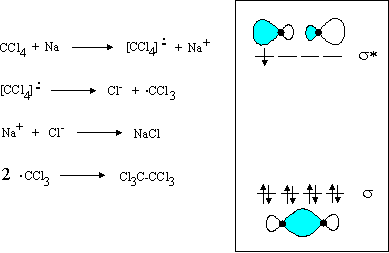











0 Response to "39 lewis diagram for ccl4"
Post a Comment