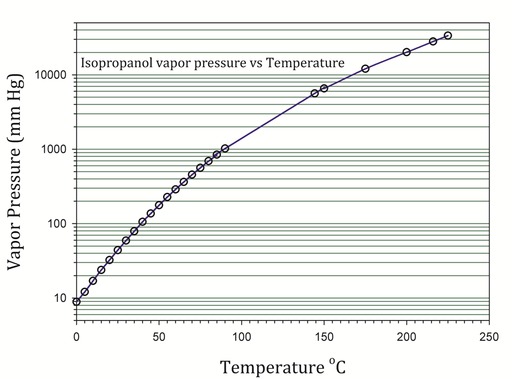
38 phase change diagram for rubbing alcohol
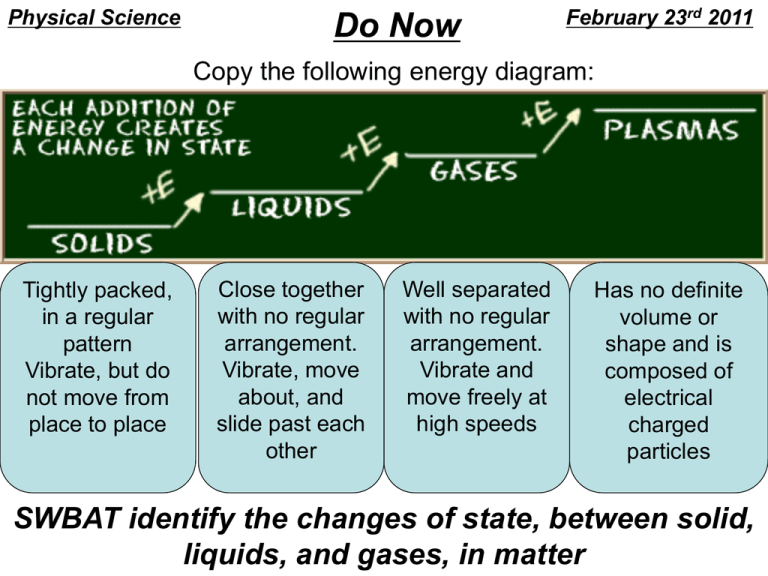
The mixture of rubbing alcohol and water has to be "perfect" in order to remain safe. Without the right amount of water, the dollar bill would burn. Knowing that, have students think about why adding water to the mixture keeps the dollar bill intact. Students should eventually determine that the water evaporates as the rubbing alcohol burns. Matter undergoes phase changes or phase transitions from one state of matter to another. Below is a complete list of the names of these phase changes. The most commonly known phase changes are those six between solids, liquids, and gasses. However, plasma also is a state of matter, so a complete list requires all eight total phase changes.
34. Refer to the phase diagram for CO2, below a. Label the four regions of the phase diagram with the appropriate phase (solid, liquid, gas, supercritical fluid). b. Indicate the approximate temperature and nature of each phase change that will occur as you heat a sample of CO2 from -100 to 40 oC at a constant pressure of 20.3 atm.
Phase change diagram for rubbing alcohol
The phase diagram of the binary system isopropyl alcohol–water was investigated by means of differential thermal analysis and powder X-ray diffraction. evaporation is a phase change that absorbs energy. During evaporation, some fast-moving, highly energetic molecules have enough energy to over-come the attractions that individual molecules have for one another and enter the gas phase. As these high-energy molecules leave the liquid phase, the average energy of the remaining liquid 34. Refer to the phase diagram for C02, below Page 9 of 12 a. Label the four regions of the phase diagram with the appropriate phase (solid, liquid, gas, d) o crow supercritical fluid). b. Indicate the approximate temperature and nature of each phase change that will occur as you
Phase change diagram for rubbing alcohol. Behaviour of pure liquids Phase diagram of a pure substance. When the temperature and pressure of a pure substance are fixed, the equilibrium state of the substance is also fixed. This is illustrated in Figure 1, which shows the phase diagram for pure argon.In the diagram a single phase is shown as an area, two as a line, and three as the intersection of the lines at the triple point, T. Description: Isopropyl alcohol is a flammable liquid with a slight odor resembling that of a ... Figure 1 shows a schematic diagram of the set-up used. level representation of the water and rubbing alcohol molecules at -6 degrees Celsius. Item 6. Using the drawings above, explain when Kellie took out the tray from the freezer, the water was still mostly solid but the rubbing alcohol was liquid. Focus your explanation on the relative strength of the electric forces between molecules. The change from liquid water to steam (a gas) is a change in phase and requires the gain of heat energy. This energy can be gained (taken in) from the environment. When you put rubbing alcohol on your skin, it makes your skin feel cold.
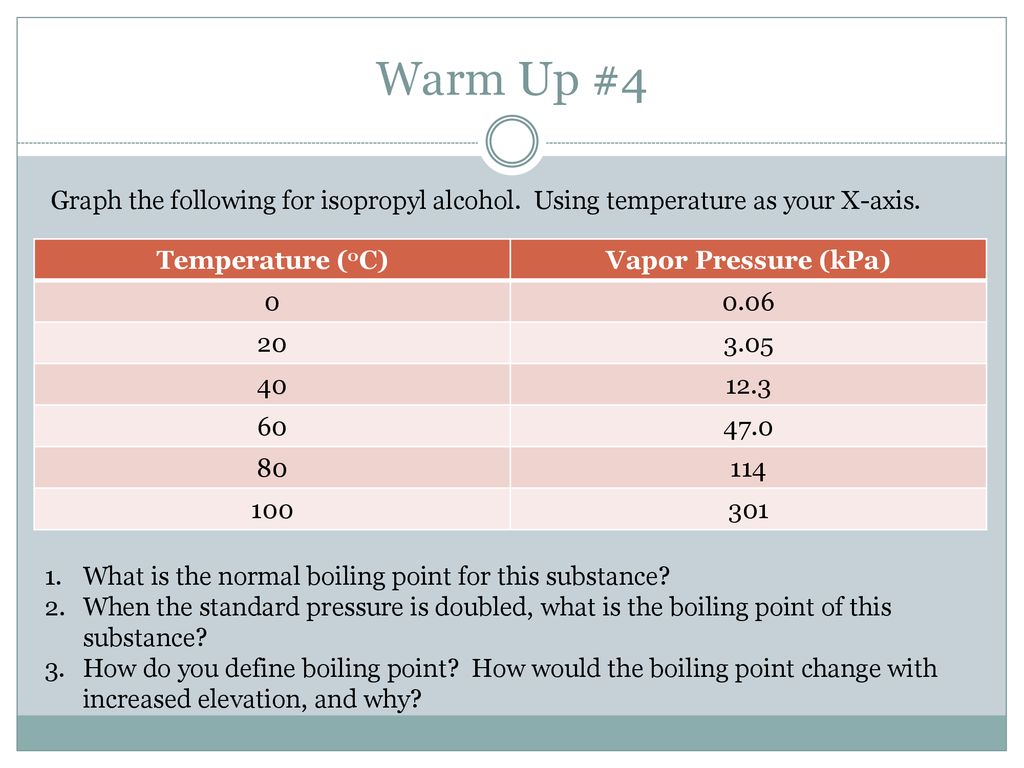
11 11.6 Phase Diagrams A.) Such a diagram allows us to predict the phase of a substance that is stable at any given temp or pressure. Phase change data · Normal boiling point · Normal melting point · Triple point temperature · Critical temperature · Critical pressure · Critical volume · Critical ...Phase change data · References In this activity, you will explore the energy change that accompanies the process of evaporation. Evaporation, like melting or freezing, is an example of a phase change—a change from one physical form of a substance to another.. During evaporation, energetic molecules leave the liquid phase, which lowers the average energy of the remaining liquid molecules. Rubbing alcohol is a product available at most pharmacies and supermarkets. One rubbing alcohol solution contains 2-propanol and water. The boiling point of 2-propanol is 82.3°C at standard pressure. Explain in terms of electronegativity differences, why a C-O bond is more polar than a C-H bond. 28.Base your answer to the following question on
37 phase change diagram for rubbing alcohol; 40 2000 cadillac deville wiring diagram; 39 black tank flush system diagram; 40 freightliner chassis wiring diagram; 41 baseball number positions diagram; 41 venn diagram of christianity islam and judaism; 39 ford 555 backhoe parts diagram; 41 refer to the diagram. in the p1p2 price range, ... 1.5 Phase Changes. A pressure cooker contains water and steam in equilibrium at a pressure greater than atmospheric pressure. How does this greater pressure increase cooking speed? As shown below, which is the phase diagram for carbon dioxide, what is the vapor pressure of solid carbon dioxide (dry ice) at [latex]-{78.5}^\circ \text{C}[/latex]? Ethanol (Ethyl Alcohol), C 2 H 5 OH, is a volatile, flammable, colorless liquid with a slight characteristic odor.It is produced via petrochemical processes or naturally by the fermentation of sugars by yeasts. Ethanol is most commonly consumed as a popular recreational drug.It is a psychoactive substance and is the principal type of alcohol found in alcoholic drinks. Phase behavior Triple point: 184.9 K (−88.2 °C), ? Pa Critical point: 508.7 K (235.6 °C), 5370 kPa Std enthalpy change of fusion, Δ fus H o: 5.28 kJ/mol Std entropy change of fusion, Δ fus S o: 28.6 J/(mol·K) Std enthalpy change of vaporization, Δ vap H o: 44.0 kJ/mol Std entropy change of vaporization, Δ vap S o: 124 J/(mol·K) Solid ...
• If you open rubbing alcohol, you can smell it. This is because the alcohol diffuses out in the _____ form. • After you recap the alcohol, an equilibrium is quickly reached again. • A dynamic EQUILIBRIUM is reached when particles are entering the gaseous phase at an equal rate as the particles go back to the liquid phase.
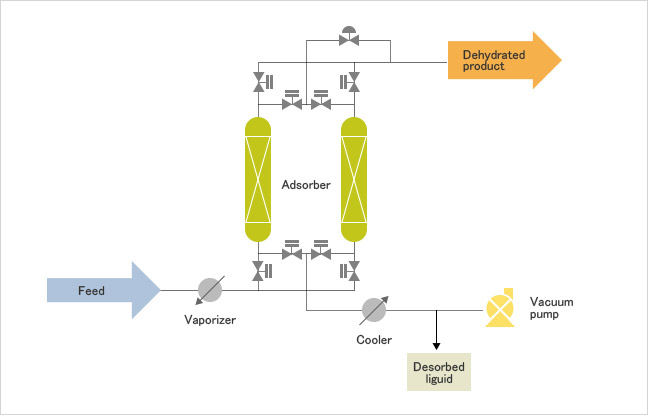
Advanced Dehydration Process For Organic Compounds Vapor Phase Psa System Technologies Gas Oil Chemicals Jgc Holdings Corporation
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist ...
Which phase change results in the release of energy? A) H20(g) B) H20(g) O, 1-120(1) C) H20(g) ... diagram the products in the given box [Indicate the exact arrangement of the particles you diagram.] ... Two alcohols that are used in our everyday lives are rubbing alcohol and ethylene glycol. Rubbing alcohol is used as an antiseptic.
Isopropyl alcohol | CH3CHOHCH3 or (CH3)2CHOH or C3H8O | CID 3776 - structure, chemical names, physical and chemical properties, classification, patents, ...
Explore how matter changes from one state to another, and visualize how temperature can measure kinetic energy. Let's get molecular with matter! Engage in experiments exploring the four states of matter, kinetic energy, and water cycles.
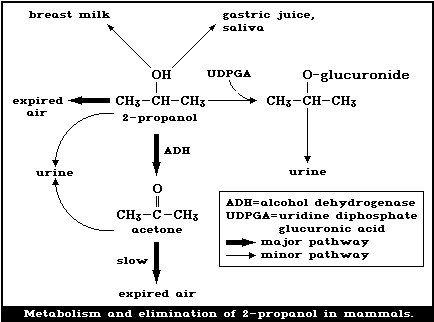
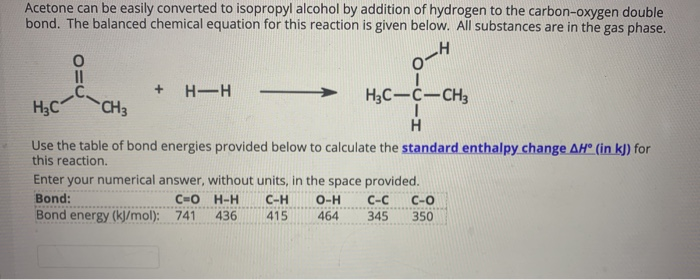
What is the change in heat when 50.0 g of isopropyl alcohol (C3H8O) melts, given that ΔHfus of isopropyl alcohol = 5.37 kJ/mol? 4.47 kJ What phase change is known as freezing?

Prediction Of Solubility Of Active Pharmaceutical Ingredients In Single Solvents And Their Mixtures Solvent Screening Intechopen
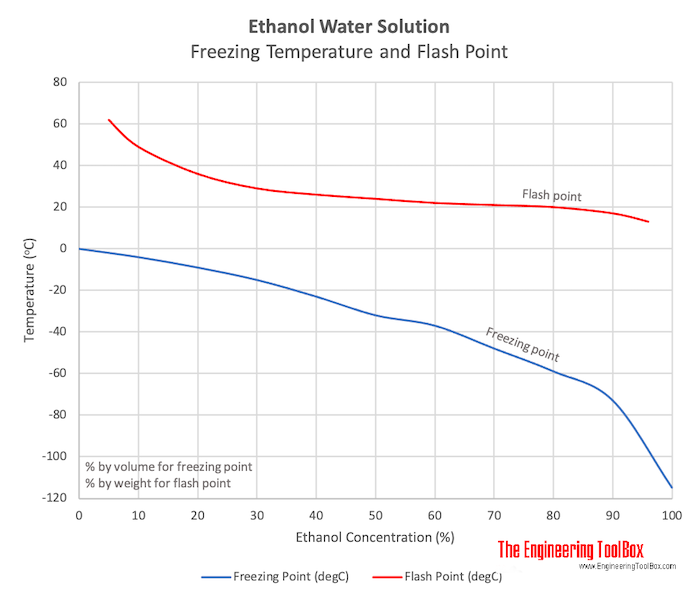
Phase change diagram for rubbing alcohol. Plug the pressure sensor into ch. Rubbing alcohol is a liquid prepared and used primarily for application. At higher altitudes the air pressure is lower. Once this phase change is complete additional energy heats the vapor to a final temperature. 1849 k 882 c.
liquids change as temperature increases? 10) Name the phase transition in each of the following situations, and indicate whether it is exothermic or endothermic: a) Bromine vapor turns to bromine liquid as it is cooled b) moth balls gradually get smaller as they sit in a drawer c) rubbing alcohol in an open container slowly disappears
Isopropyl Alcohol. Formula: C 3 H 8 O. Molecular weight: 60.0950. IUPAC Standard InChI: InChI=1S/C3H8O/c1-3 (2)4/h3-4H,1-2H3. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: KFZMGEQAYNKOFK-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet.
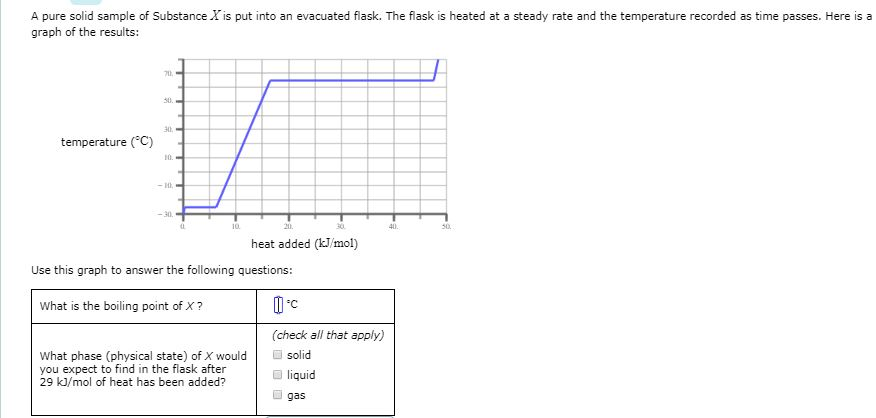
Looking at the phase change diagram for water and following the dashed line at 1 atm, you can see that water would begin as a solid (ice) and melt at 0ºC. All of the water would be in liquid form by the time the temperature reached 75ºC. The second type of phase change graph you might see on the SAT II Chemistry exam is called a heating curve.
Inthis cycle certain changes with the componets are made which are as follows: 2.1. Phase Changer As the name suggests, a Phase changer (Fig.2) is device in which the phase of alcohol is changed from liquid to gaseous form. In this system, a closed tank is used to make a chamber for hot water, there is a copper pipe coming from the pump,
at 30 and 2.204. diagonal, liquid phase. 2,856, top horizontal line. flat, the change from a solid to a liquid. What does the diagram show about phases and the phase of the substance as it is heated? Check all that apply. The particles are close together but move in different directions between 0°C and 2,200°C.
Rubbing alcohol is a product available at most pharmacies and supermarkets. One rubbing alcohol solution contains 2-propanol and water. The boiling point of 2-propanol is 82.3°C at standard pressure. 71 Explain, in terms of electronegativity differences, why a C O bond is more polar than a C H bond. [1]
34. Refer to the phase diagram for C02, below Page 9 of 12 a. Label the four regions of the phase diagram with the appropriate phase (solid, liquid, gas, d) o crow supercritical fluid). b. Indicate the approximate temperature and nature of each phase change that will occur as you
evaporation is a phase change that absorbs energy. During evaporation, some fast-moving, highly energetic molecules have enough energy to over-come the attractions that individual molecules have for one another and enter the gas phase. As these high-energy molecules leave the liquid phase, the average energy of the remaining liquid
The phase diagram of the binary system isopropyl alcohol–water was investigated by means of differential thermal analysis and powder X-ray diffraction.

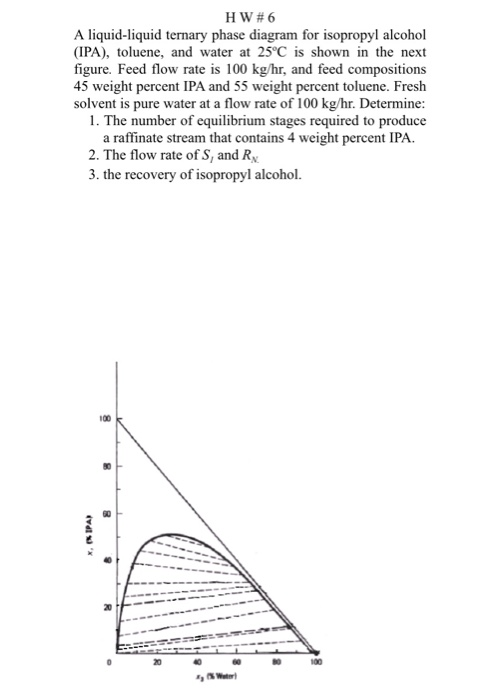
Liquid Liquid Solid Equillibrium Of Water 2 Propanol Kosmotropic Salts Construction Of Phase Diagrams And Understanding Of Salting Out Effects Using Volumetric And Compressibility Studies Bentham Science

Conversion Of A Surfactant Based Microemulsion To A Surfactant Free Microemulsion By Co 2 Soft Matter Rsc Publishing Doi 10 1039 C8sm02444h






(82).jpg)







0 Response to "38 phase change diagram for rubbing alcohol"
Post a Comment