38 orbital diagram for sodium
Draw Orbit Structure Diagram of Sodium Chloride (Nacl) CISCE ICSE Class 9. Question Papers 10. Textbook Solutions 19257. Important Solutions 6. Question Bank Solutions 14520. Concept Notes & Videos 431. Syllabus. Advertisement Remove all ads. Draw Orbit Structure Diagram of Sodium Chloride (Nacl) - Chemistry ... Click here👆to get an answer to your question ️ 9. Draw orbit structure diagram of sodium chloride (NaCl) and calcium oxide (Cao).
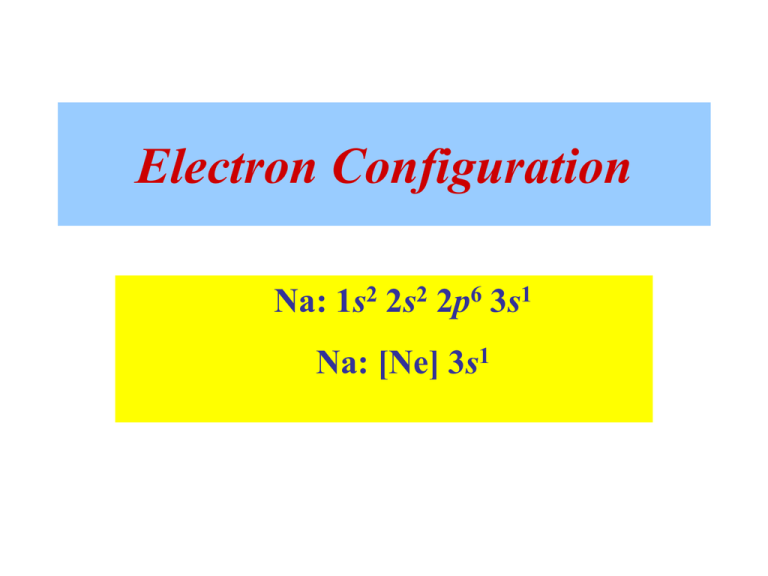
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.

Orbital diagram for sodium
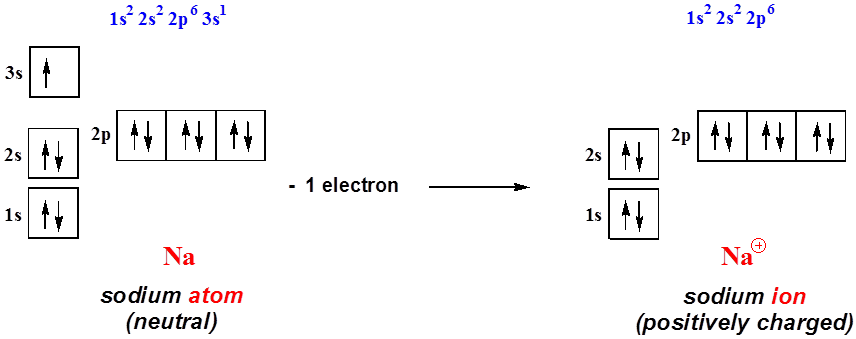
For example, the abbreviated electron configuration for sodium is written as [Ne]3s1, where [Ne] represents the core electrons in the 1s, 2s, and 2p subshells. Which element will not have an abbreviated electron configuration beginning with a noble gas configuration? In your response, use the elemental symbol. The orbital diagram provided ... Ionic bonding Sodium is an alkali metal, and chlorine is a halogen. This means that sodium contains one electron in its outer orbital and chlorine contains seven electrons in its outer orbital. For example, sodium (Na), which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon.
Orbital diagram for sodium. Combine the two sodium valence atomic orbitals to produce bonding and antibonding molecular orbitals. Draw the molecular orbital energy-level diagram for this system. Determine the total number of valence electrons in the Na 2 − ion. Fill the molecular orbitals in the energy-level diagram beginning with the orbital with the lowest energy. Draw orbit structure diagram of Sodium chloride (NaCl) Medium. View solution. >. Which of the following is the correct electron dot structure of N 2. . Chem4Kids.com: Sodium: Orbital and Bonding Info. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table . In the next section we're going to cover electron orbitals or electron ... 1 thought on " Electron Configuration and Orbital Box Diagram for First Excited State of Sodium " Habib Alkhaldi December 10, 2014 at 2:34 pm. Thank you
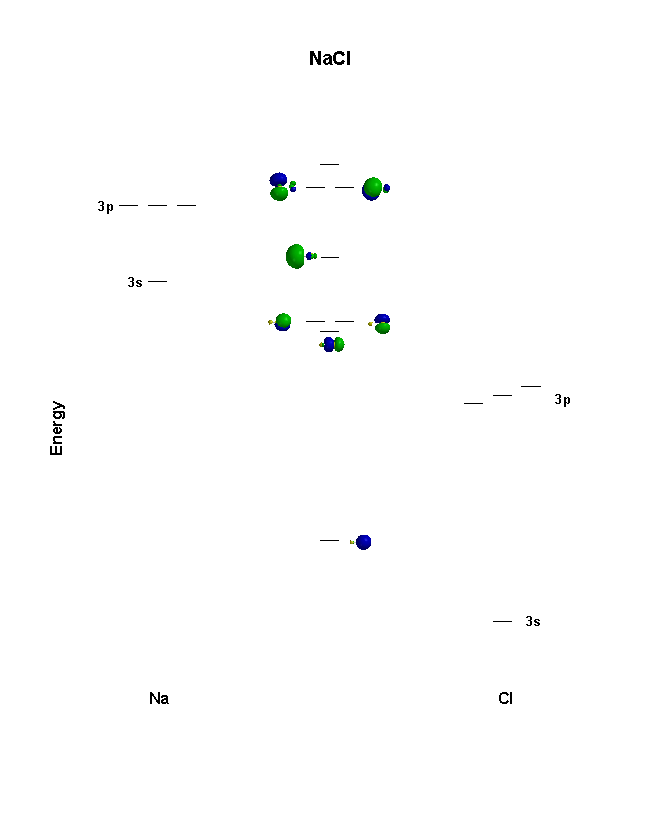
As in the case of the second level the 3s orbital is lower in energy than the 3p which is lower in energy compared to the 3d. So as we progress from sodium across the period to argon the electrons are placed in the orbitals just as they were for the second period. The orbital diagrams for the eight elements are shown below. This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand nobl... Example: NaCl. In NaCl, the sodium \(3s\) orbital (-5.2 eV) is significantly higher in energy than the chlorine valence orbitals. The chlorine \(3s\) and \(3p_z\) orbitals have compatible symmetry, yet only the \(3p_z\) orbital (-13.8 eV) is close enough in energy to interact with the Na \(3s\); still the energy difference is large enough to make bonding weak. In this video, we determine how to draw the orbital diagram of sodium.
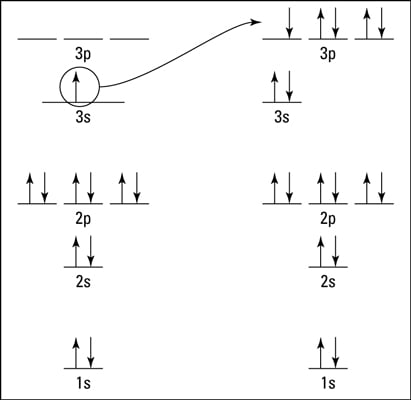
Sodium Spectrum The sodium spectrum is dominated by the bright doublet known as the Sodium D-lines at 588.9950 and 589.5924 nanometers. From the energy level diagram it can be seen that these lines are emitted in a transition from the 3p to the 3s levels. The line at 589.0 has twice the intensity of the line at 589.6 nm. Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. Two unpaired electrons. Write the electron configuration for Ge. Orbital Diagram. 1s ... There are few uses for the pure metal, however its compounds are used in medicine, agriculture and photography. Sodium chloride (NaCl) is table salt. Liquid sodium is sometimes used to cool nuclear reactors. Sources Obtained by electrolysis of melted sodium chloride (salt), borax and cryolite. Hydrogen (H) Electron Configuration with Full Orbital Diagram. Hydrogen electron configuration is 1s 1. Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles.
The electronic configuration of Sodium (atomic number is 11) is- 1s^2 2s^2 2p^6 3s^1 Note:- For writing the electronic configuration of elements, the Aufbau Principle is used. In Aufbau Principle, the electrons are filled according to the increasing energy level of orbitals. According to the Aufbau Principle, first the atomic number of element is determined (like here oxygen has atomic number ...
The orbital diagram of the sodium atom is shown below. The sodium atom has 11 electrons which are contained in 1s, 2s, 2p and 3s orbitals. Each s...
The groundstate for Sodium (11-Na) is: 1S2 , 2S2, 2P6, 3S1 If you count the ^powers you notice it'll sum to 11, when Sodium is excited the outermost electron (3S1) will be excited from the 3S ...

11 Na Sodium Electron Shell Structure Schoolmykids Matter Worksheets Element Chemistry Electron Configuration
So, for example, if we wanted to know the electron configuration for sodium (atomic number 11), we start at the top left and follow that arrow to 1s2 (we can only add two electrons to an "s" orbital).(See orbital list in the lower right of the graphic). Following the next arrow, we fill another "s" orbital 2s2.
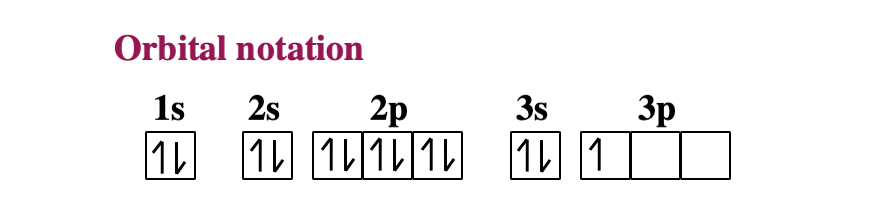
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Orbital Diagrams •Orbital diagram for sodium. •e-1config: 1s2 2s2 2p6 3s 1s 2s 2p 3s . Orbital Diagrams •Orbital diagram for magnesium. •e-2config: 1s2 2s2 2p6 3s 1s 2s 2p 3s . Orbital Diagrams
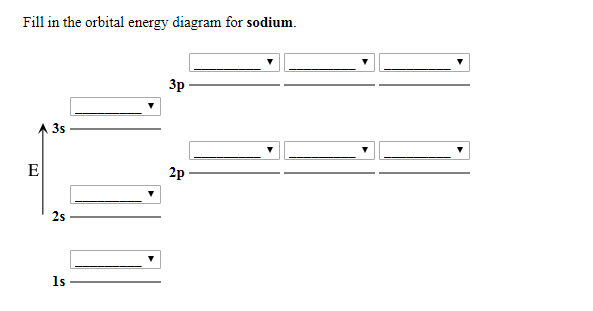
Chemistry questions and answers. 1 attempts left Check my work Select the single best answer. Choose the correct orbital diagram for the ground-state electron arrangement for sodium. Be sure to follow the three orbital filling rules: the aufbau principle, the Pauli exclusion principle, and Hund's rule. 1s 2s 2p 3s 1s 2s 2p 3s 3p 1s 2s 2p 3s 1s ...
The eleventh electron in Sodium will enter the 3s orbital. Therefore, the electronic configuration of Sodium can also be written in a condensed form as [Ne] 3s ¹. Following table gives condensed form for elements from Sodium to Potassium.
Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining electron in the 3s. Therefore the sodium electron configuration will be 1s 2 2s 2 2p 6 3s 1.
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ...
For example, sodium (Na), which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon.
Ionic bonding Sodium is an alkali metal, and chlorine is a halogen. This means that sodium contains one electron in its outer orbital and chlorine contains seven electrons in its outer orbital.
For example, the abbreviated electron configuration for sodium is written as [Ne]3s1, where [Ne] represents the core electrons in the 1s, 2s, and 2p subshells. Which element will not have an abbreviated electron configuration beginning with a noble gas configuration? In your response, use the elemental symbol. The orbital diagram provided ...

Electron Configurations And Orbital Diagrams Principles For Filling Orbitals Writing Electron Configurations Ppt Download

Select The Correct Statement Below A Phosphorous Contains 10 Core Electrons And 5 Valence Brainly Com

High School Chemistry Electron Configurations Of Main Group Elements Wikibooks Open Books For An Open World



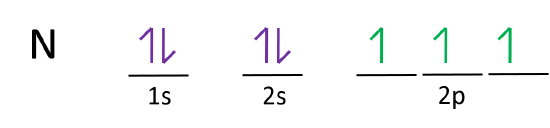
















0 Response to "38 orbital diagram for sodium"
Post a Comment