38 miscibility gap phase diagram
for low temperatures, there exists a miscibility gap separating two compositionally different solid solutions, a Na-rich and a K-rich one. Here, we have predicted the Na-rich/K-rich phase envelope in the solid part of the phase diagram using molecular simulation. The calculated phase diagram reproduces the experimental phase dia- The miscibility gap at low temperatures can be understood with a regular solution model. Figure by MIT OCW. 800 700 600 500 400 300 200 100 0 10 20 30 40 50 60 70 80 90 100 Alpha Liquid Beta Atomic Percent Lithium T (temperature) o C Al Ll Alpha Beta Phase diagram for light metals Aluminum-Lithium.
To some extent, rare-earth-doped UO(2) is representative of an irradiated nuclear fuel. The two phases we observed previously in neodymium-doped UO(2) are now interpreted as the existence of a miscibility gap in the U-Nd-O phase diagram using new results obtained with Raman spectroscopy. Extrapolati …
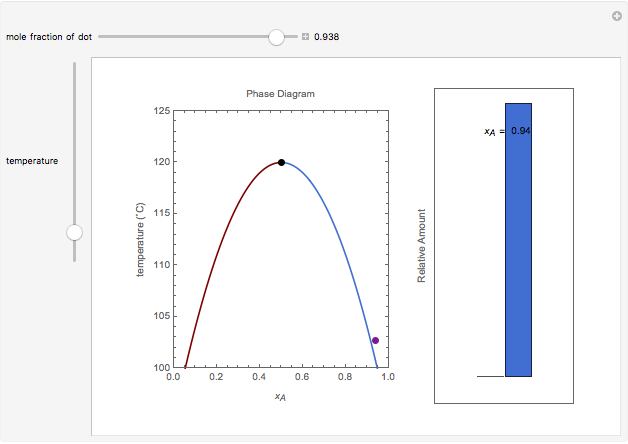
Miscibility gap phase diagram
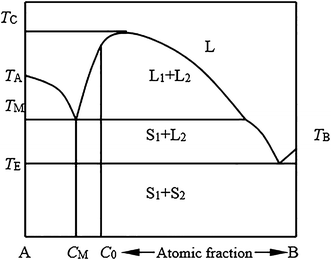
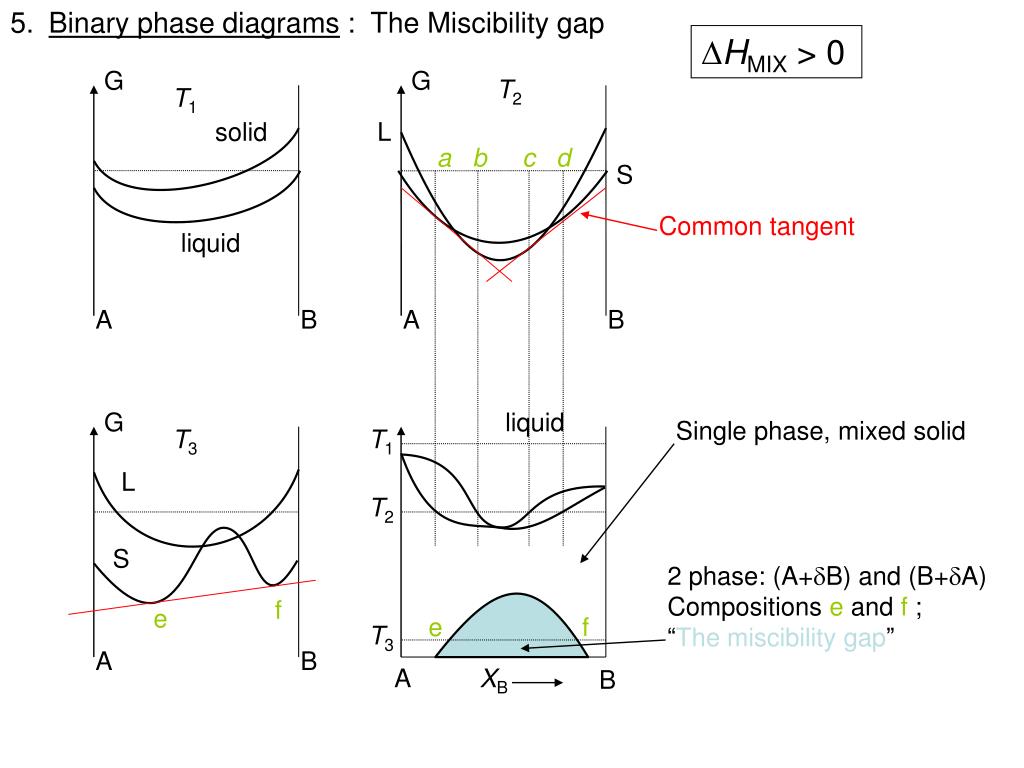
Fig. 3 Schematic binary phase diagrams with solid state miscibility where the liquidus shows a maximum (a) and a minimum (b). It also is possible to have a gap in miscibility in a single-phase field; this is shown in Fig. 4. Point Tc, above which phase α1 and α2 become indistinguishable, is a critical point. Lines a-Tc and b-Tc, called solvus ... Phase Diagrams Are Derived from Free Energy Curves Immiscibility Dome or Miscibility Gap one liquid two liquids. Cer103 Notes Shelby Chapter 4 4-5 R.K. Brow Immiscibility/Phase Separation Mechanisms for Phase Separation: 1. Nucleation and Growth • Analogous to nucleation/crystallization cibility gap within the chalcopyrite phase and a small miscibility gap between the chalcopyrite and the zinc-blende phases). The diagram of case (ii) shows the role of positive K values: it widens the CH-ZB miscibility gaps, suppressing the small second-order transition line present in case (i) between — 410 and 550 K, and re-moves the triple ...
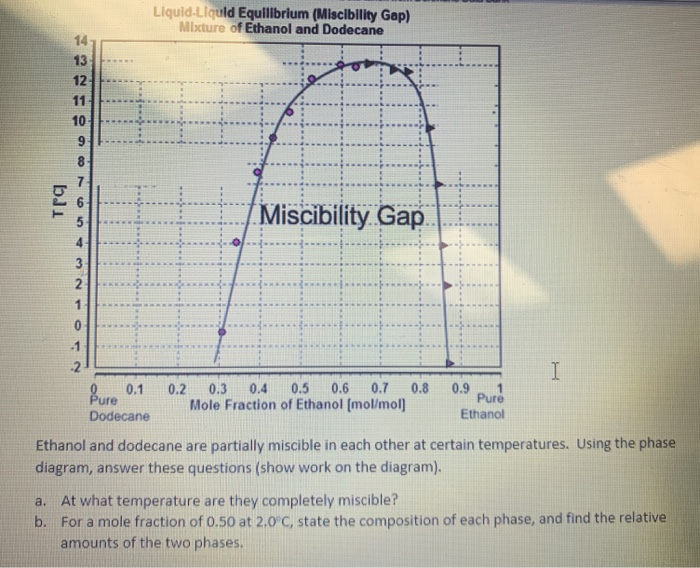
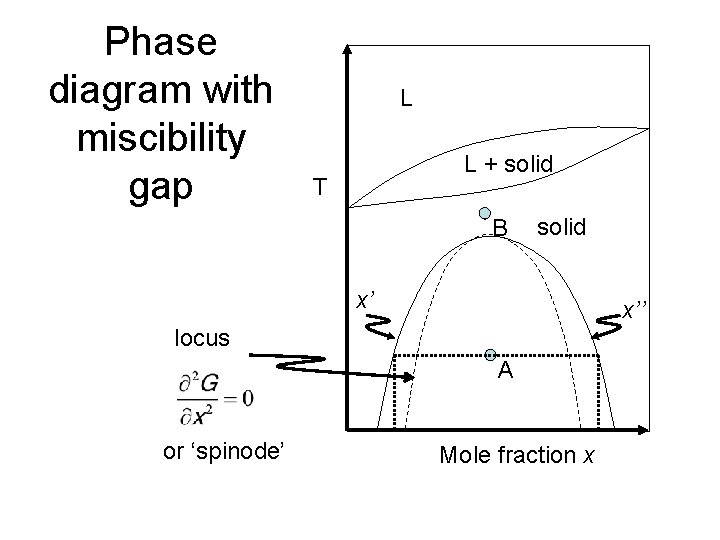
Miscibility gap phase diagram. Lecture 8: Phase Diagrams 1. Learning objectives 2 Phase diagrams Be able to read a phase diagram Be able to identify the phases present at a given point Be able to calculate how much of those phases there are Be able to predict the microstructures from the phase diagram 3 Mixing Understand the thermodynamic basis of mixing Understand the origin of the miscibility gap Be able to use the phase ... Hiroaki Okamoto, Thaddeus B. Massalski, in Methods for Phase Diagram Determination, 2007. 3.12 Displaced Miscibility Gap (Point q in Fig. 3.3). The composition of the critical point of a liquid miscibility gap is usually found to occur in the center range of a phase diagram [7].A binary phase diagram having a liquid miscibility gap that is significantly displaced toward either end of the ... A miscibility gap is a region in a phase diagram for a mixture of components where the mixture exists as two or more phases – any region of composition of mixtures where the constituents are not completely miscible.. The IUPAC Gold Book defines miscibility gap as "Area within the coexistence curve of an isobaric phase diagram (temperature vs composition) or an isothermal phase diagram ... 1. Free energy diagrams define the structure of the phase diagram. 2. Compositions of two-phase regions, which are tangent points on the free energy diagram, become phase boundaries on the phase diagram. 3. The lever rule is directly applied to tie lines on the phase diagram, to determine the amount of each phase present in two-phase regions.
cibility gap within the chalcopyrite phase and a small miscibility gap between the chalcopyrite and the zinc-blende phases). The diagram of case (ii) shows the role of positive K values: it widens the CH-ZB miscibility gaps, suppressing the small second-order transition line present in case (i) between — 410 and 550 K, and re-moves the triple ... Phase Diagrams Are Derived from Free Energy Curves Immiscibility Dome or Miscibility Gap one liquid two liquids. Cer103 Notes Shelby Chapter 4 4-5 R.K. Brow Immiscibility/Phase Separation Mechanisms for Phase Separation: 1. Nucleation and Growth • Analogous to nucleation/crystallization Fig. 3 Schematic binary phase diagrams with solid state miscibility where the liquidus shows a maximum (a) and a minimum (b). It also is possible to have a gap in miscibility in a single-phase field; this is shown in Fig. 4. Point Tc, above which phase α1 and α2 become indistinguishable, is a critical point. Lines a-Tc and b-Tc, called solvus ...
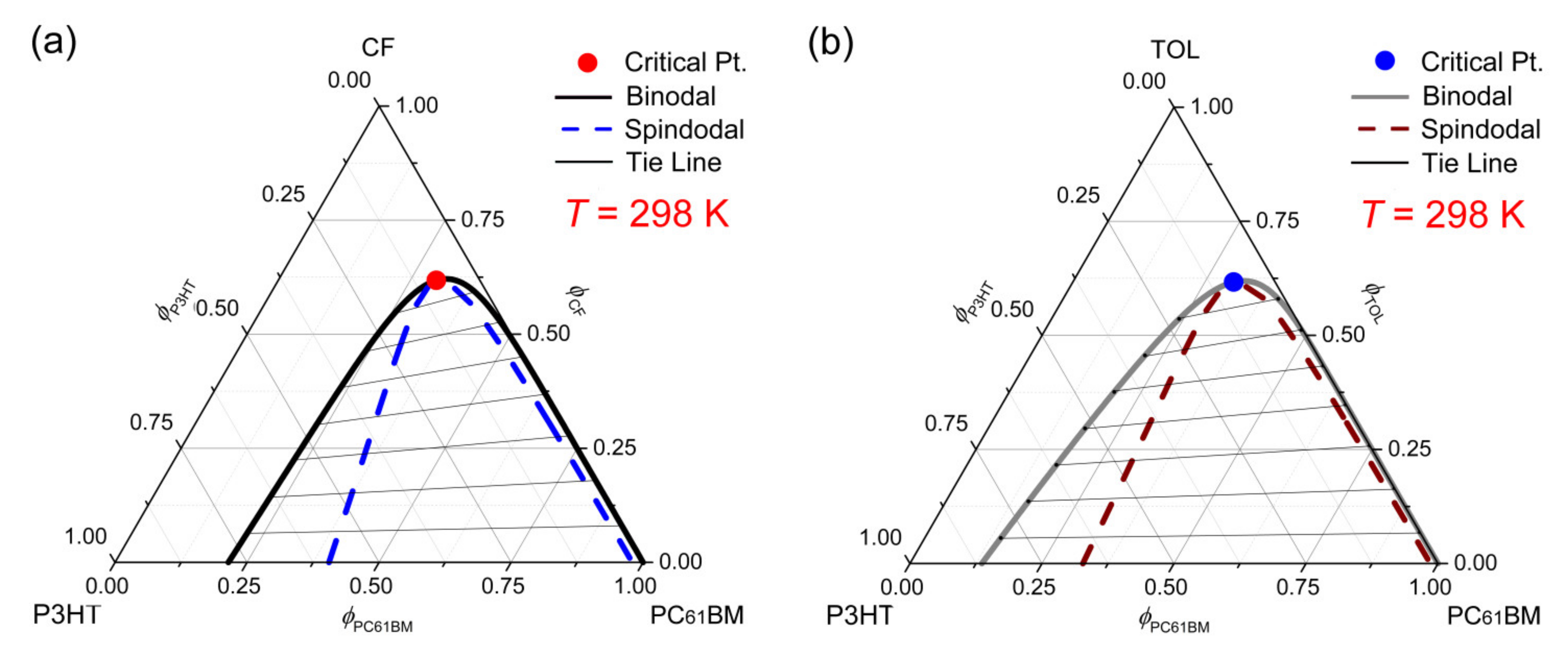
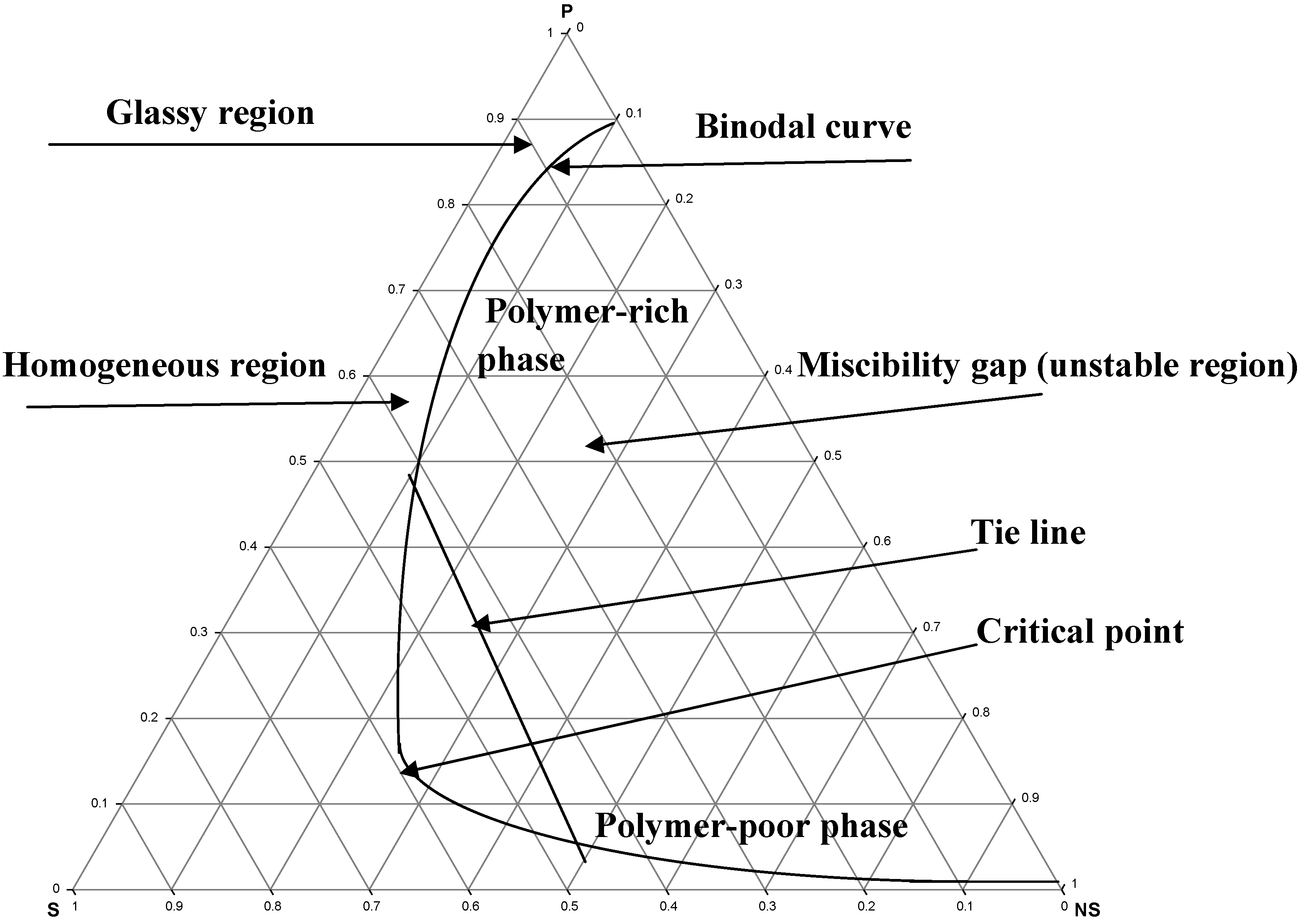
Polymers Free Full Text Phase Diagrams Of Ternary P Conjugated Polymer Solutions For Organic Photovoltaics Html

Ab Initio Prediction Of Low Temperature Phase Diagrams In The Al Ga In As System Mas M As M M Al Ga Or In And Alas Gaas Inas Via The Global Study Of Energy Landscapes Physical Chemistry Chemical
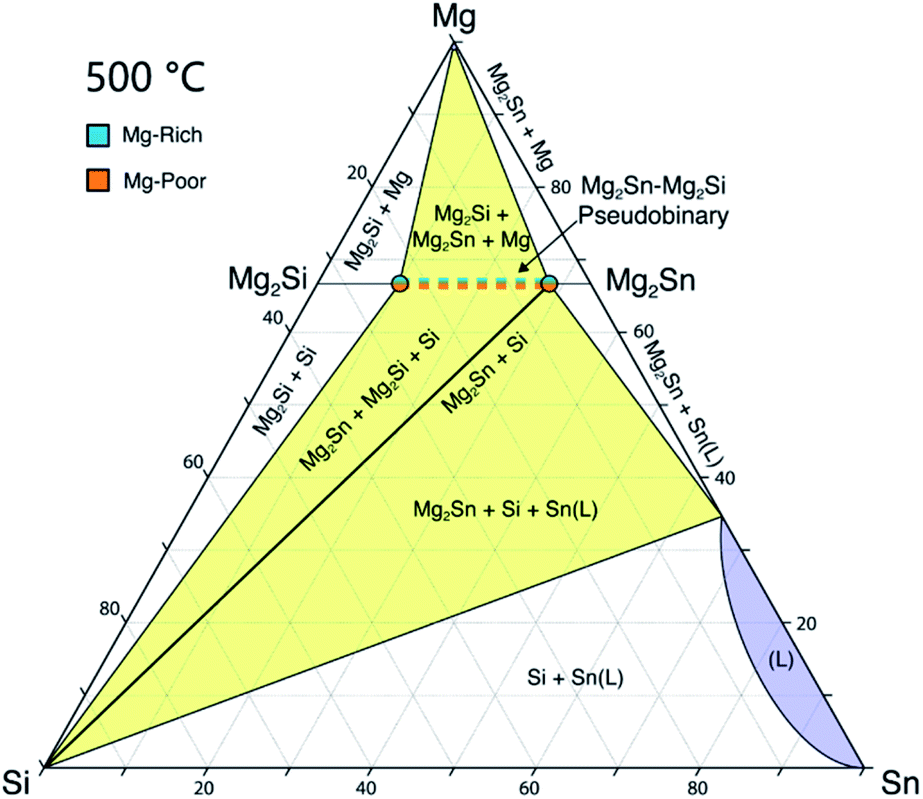
Using Phase Boundary Mapping To Resolve Discrepancies In The Mg 2 Si Mg 2 Sn Miscibility Gap Journal Of Materials Chemistry A Rsc Publishing Doi 10 1039 D1ta00115a
















0 Response to "38 miscibility gap phase diagram"
Post a Comment