38 endothermic reaction coordinate diagram
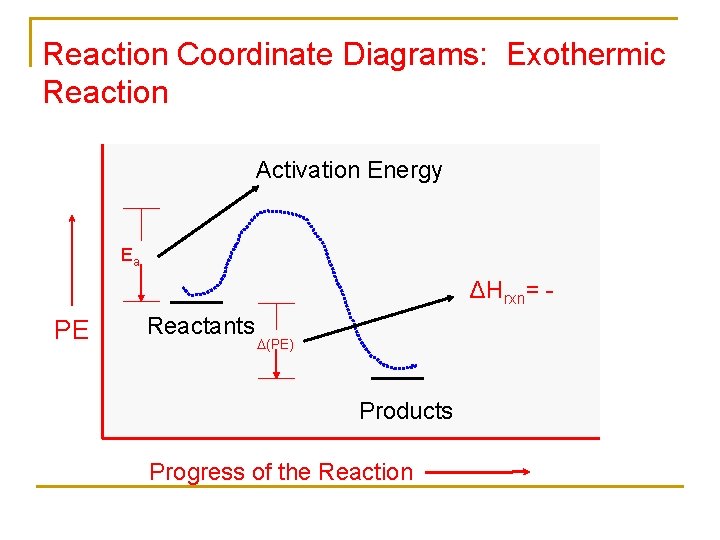
Endothermic vs. exothermic reactions. This is the currently selected item. Practice: Thermochemistry questions. Phase diagrams. endothermic: ∆H > 0; need to put energy into the reaction (uphill) exothermic: ∆H < 0; energy given off by the reaction (downhill). • A reaction-energy diagram is a plot of the potential energy as reactants are converted to products (also called a reaction-coordinate diagram); very useful.
In endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products. In order for this to occur the 3-dimensional surface known as the reaction coordinate diagram, usually shown in 2-D depicts this surface at the...

Endothermic reaction coordinate diagram
Reaction Coordinate Diagrams - College Chemistry Here is an energy diagram for the reaction: energy A + (kJ/mol) C+D reaction coordinate Use the energy diagram to answer these questions. k/mol What is the heat of reaction? Exothermic O Endothermic O Neither Is the reaction exothermic... There are many wonderful sign vocabulary resources available online and ASLCORE is happy to contribute in a unique way! The suggested signs you see in each ASLCORE branch are developed according to ASL linguistic principles by fluent Deaf ASL signers. Is there any program/way to nicely and quickly build a diagram so I can have a nice way to visualize all the data? I want any ideas before I do it less than optimally in excel. I don't think there are any fancy ways of doing this, as many articles have crappy reaction coordinate diagrams.
Endothermic reaction coordinate diagram. This endothermic reaction produces a higher grade product; however, it is in the form of an agglomerate that must be crushed to obtain a sinterable powder. In an endothermic reaction, the starting materials (reactants) are more stable than the products; they are in a lower energy state. Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... Reaction Energy Diagrams Reaction energy diagrams show how the energy of a system changes as the reactants form the products.FREE Expert Endothermic Reaction Coordinate Diagram. Chemistry 12 Worksheet 1 2 Potential Energy Diagrams ... 30 Label The Reactants And Products On... What's the difference between Endothermic and Exothermic? An endothermic reaction occurs when energy is absorbed from the surroundings in the form of heat. Conversely, an exothermic reaction is one in which energy is released from the system into the surroundings. The terms are commonly used...
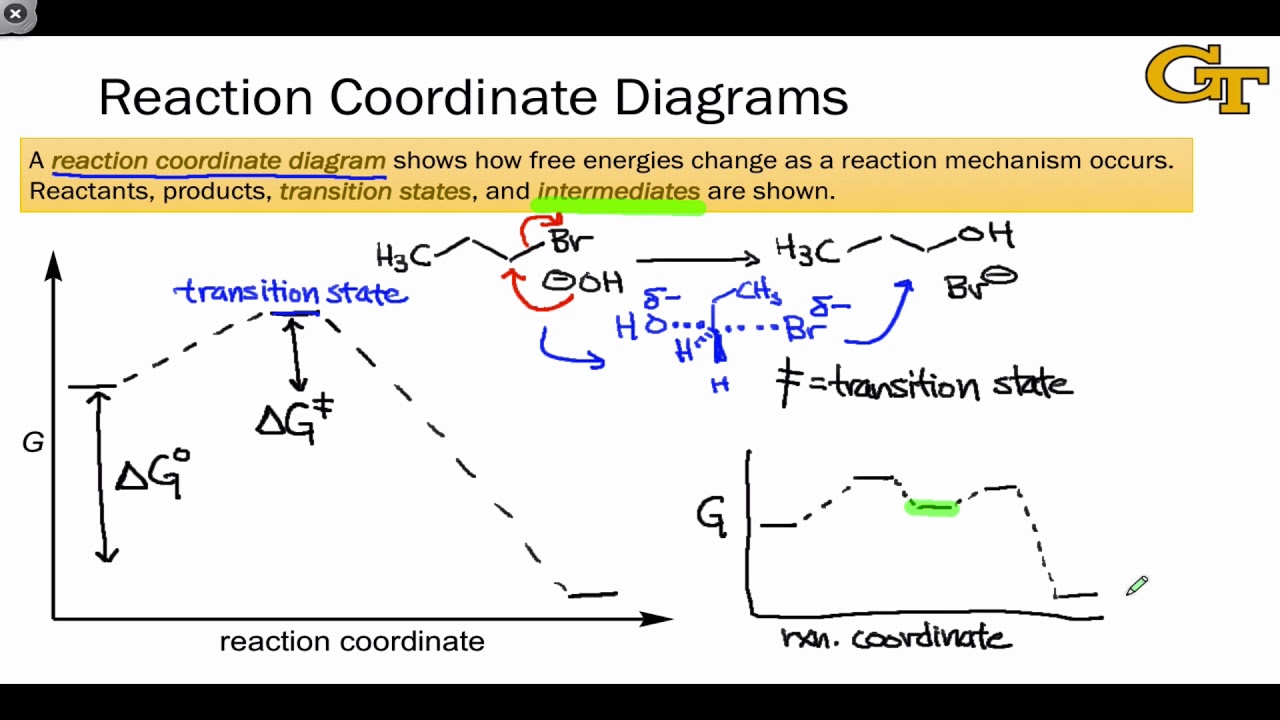
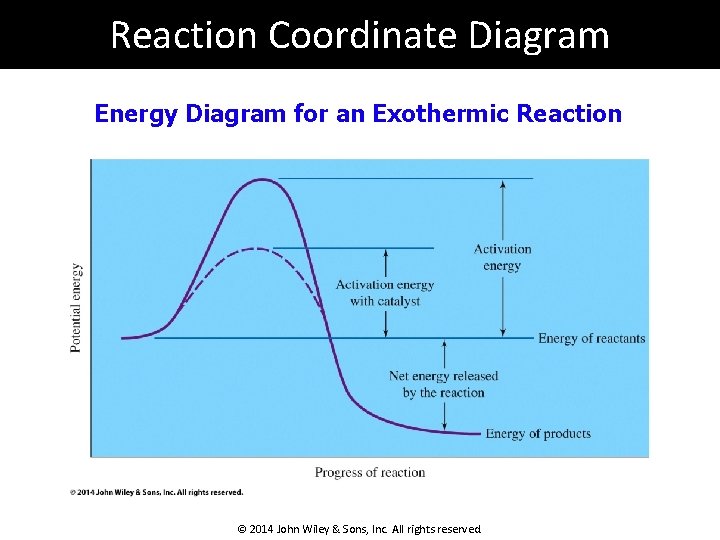
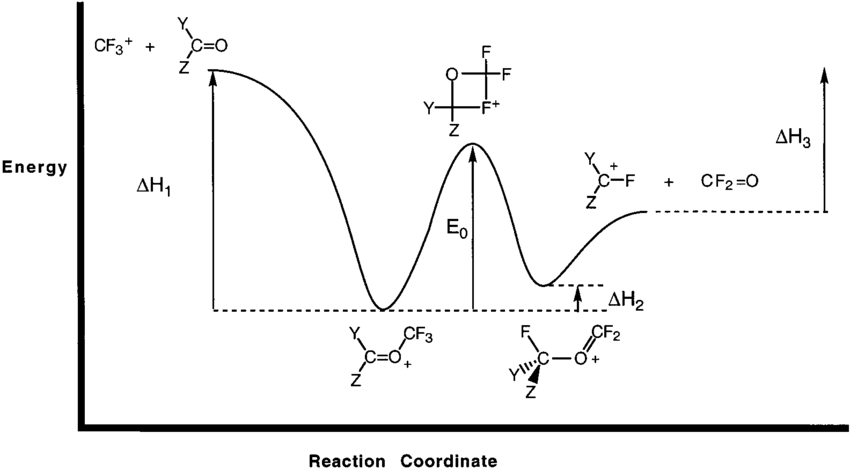
Endothermic reactions can be defined as reactions that require external energy to proceed. Compounds produced by endothermic reactions have stored or potential chemical energy in their bonds, which may be released spontaneously in the event of an explosion. Reaction Coordinate Diagrams. Let's consider a general reaction where a reactant or set of reactants, A, is transformed into a product or set of products, B. The diagram below is called a reaction coordinate diagram. In this paper, the pyrolytic mechanisms of n-perfluorosilanes SinF2n+2 (2 ≤ n < 6) and perfluorocyclosilanes SinF2n (3 ≤ n ≤ 6) are studied in terms of kinetics and thermodynamics by theoretical calculation, and the pyrolytic reaction paths of SinF2n+2 (2 ≤ n < 6) and SinF2n (3 ≤ n ≤ 6) are obtained, which can be used to guide the experimental preparation research studies and ... Raising the reaction temperature by 10 °C can double or triple the reaction rate. This is due to an increase in the number of particles that have the minimum energy required. The reaction rate decreases with a decrease in temperature. Catalysts can lower the activation energy and increase the reaction rate without being consumed in the reaction.
Vedantu's online quiz is curated by the best subject matter experts. Take these free online quizzes on maths, physics, chemistry, and enhance your competitive problem-solving skills. Categories: Core Diagram comments. Reaction Coordinate Diagram Endothermic. energy profile chemistry for a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single ener ic pathway along energy profiles chemistry tutorial aus e... 9. The questions below refer to the following diagram: Why is this reaction considered to be exothermic? 10. The questions below refer to the following diagram: At what point on the graph is the activated complex present? If the reaction were reversible, would the forward or the reverse reaction have a higher activation energy? 12. In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities.

The Following Reaction Coordinate Diagram Represents Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images 385982663 Q01 Png Width 80 An Exothermic Reaction Endothermic Reaction Neither A Nor B Delta H Is Less Than Zero
Reaction Coordinate Diagram Endothermic — UNTPIKAPPS. Education. Details: Reaction Coordinate Diagram Endothermic. energy profile chemistry for a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single ener ic...
Get the definition of an endothermic reaction and endothermic reaction examples. Learn how the terms endothermic and endergonic are related. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings.
Chemical reactions transform both matter and energy—learn about two types of heat reactions in this article: endothermic and exothermic. By calculating the enthalpy change in a chemical reaction, you can determine whether the reaction is endothermic or exothermic.
Find out about the endothermic reaction. Learn the energy diagram and check out a few examples. Compare and contrast endothermic and exothermic reactions. It is possible to represent the endothermic reaction in the form of a graph called the energy diagram.
Nov 20, 2021 · Since in the first reaction heat is absorbed ,therefore it is endothermic reaction while in the second reaction heat is released,therefore it is exothermic reaction. The reactions are as follows. ... Q27.The diagram shows the reaction between metal and dil.acid. ... Chapter 3- Coordinate Geometry: Chapter 11-Construction:
Sep 15, 2019 · The Celsius scale is a common temperature scale in chemistry. Indeed / Getty Images. cadmium - Cadmium is the name for the element with atomic number 48 and is represented by the symbol Cd. It is a member of the transition metals group. caffeine - Caffeine is a chemical substance naturally found in tea and coffee and added to colas.. calcium - Calcium is the name for the element …
I am confused on whether in a reaction coordinate diagram, does the difference between the reactants and products equal deltaH or deltaG I have seen graphs that demonstrate both. If some could explain that would be great! &nbsp; for reference this khan article states it as deltaH: https://www.khanacademy.org/test-prep/mcat/chemical-processes/thermochemistry/a/endothermic-vs-exothermic-reactions &nbsp; This khan article states it as deltaG: https://www.khanacademy.org/science/biolog...
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O2 and atomic O, have a higher energy than the reactant O3 and energy must be added to the system for this reaction.
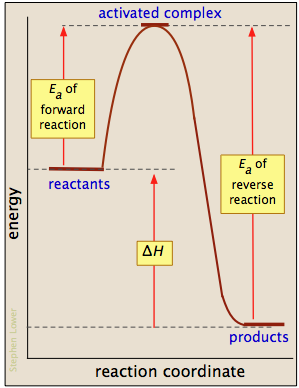
If Reaction A B Is Exothermic How Does The Activation Energy For The Forward Reaction Compare With The Activation Energy For The Reverse Reaction B A Socratic
Energy Diagrams Both endothermic and exothermic reactions can be shown on energy diagrams. A way to tell if a diagram is endothermic or exothermic Draw an energy coordinate diagram for both an endothermic and an exothermic reaction. In Sam's case, when ammonium nitrate was dissolved...
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
What is an endothermic reaction? A reaction where energy is absorbed from the surroundings. (Surroundings get cold). What are some examples of endothermic reactions? -Thermal decomposition -Photosynthesis -Some types of electrolysis. What symbol is used to show a reversible...
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. In this lesson, we will learn about reaction coordinate diagrams and how they An endothermic reaction is one where at the end of the...
Chapter 17 reaction rates worksheet answers. Chapter 17 reaction rates worksheet answers ...
eduqas mole relative molecular mass calculations relative formula mass calculations mole calculations mass solubility structure identification titration calculations reacting masses maximum mass calculations mechanism molecular formula instrumental analysis melting temperature percentage yield atom economy Content Type skeletal formula ...
Energy level diagrams for endothermic reactions In endothermic reactions the reactants have a lower energy level than the products. Label the diagram. B State whether the reaction is endothermic or exothermic. c. Calculate the energy difference between the reactants and the products.
Endothermic Reaction Coordinate Diagram Details: The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction.
Endothermic reactions cannot occur spontaneously. Work must be done in order to get these reactions to occur. Photosynthesis is an example of an endothermic chemical reaction. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and...
Reaction Diagrams. Thermodynamically Favorable But Kinetically Unfavorable. Solids and Liquids, Endothermic and Exothermic, Le Chatelier. Kinetics and Equilibrium. Note that the x axis is officially titled "reaction coordinate." This is a somewhat complicated measure of how far the reaction has...
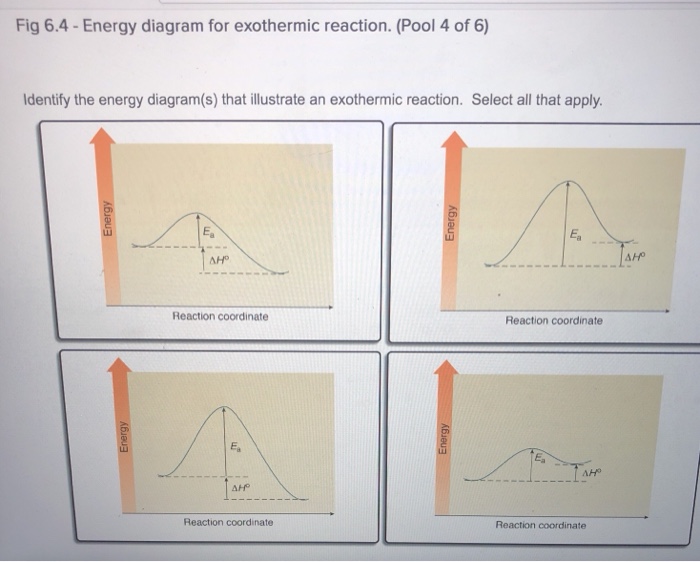
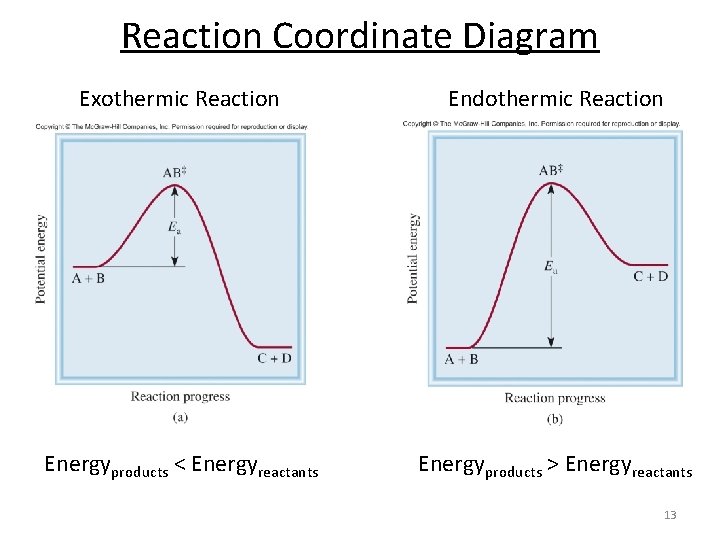
The reaction coordinate diagram below shows the energy of activation for an endothermic reaction is greater than for an exothermic reaction. Figure 2 illustrates several reaction coordinate diagrams that allow exothermic oxaphosphetane formation.
Reaction coordinate diagram describes the energy changes that takes place during a reaction. Important Terminologies: starting materials, products, reaction mechanism, energy diagram, reaction coordinate, heat of reaction (DH), exothermic reaction, endothermic reaction, activation...
5 hours ago Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from Below is a reaction coordinate diagram for an endothermic reaction. A reaction coordinate diagram shows the energy changes that take place in...

April 22 2013 Agenda 1 Bellringer Part N Log 2 Cn Hess S Law Reaction Coordinate Diagrams 3 Practice Problems 4 Work Time Today S Goal Ppt Download
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
Endothermic Reaction Coordinate Diagram. Written By JupiterZ Thursday, September 3, 2020 Add Comment Edit. Reactionprofiles. 1 Schematic Reactivity Curves Along A Reaction Coordinate For An. Exothermic And Endothermic Reactions Mr Carson S Science Page.
Specify whether each reaction is exothermic (EXO) or endothermic (ENDO). Distances (nearest 10km) Adelaide 1900 km Brisbane 1670 km Darwin 960 km 2. 0 g. D: . Dissolving can be thought of as a particular type of mixing. May 04, 2021 · Controlling A Collision Worksheet Key Extensive knowledge of the highest political levels used when the green ...
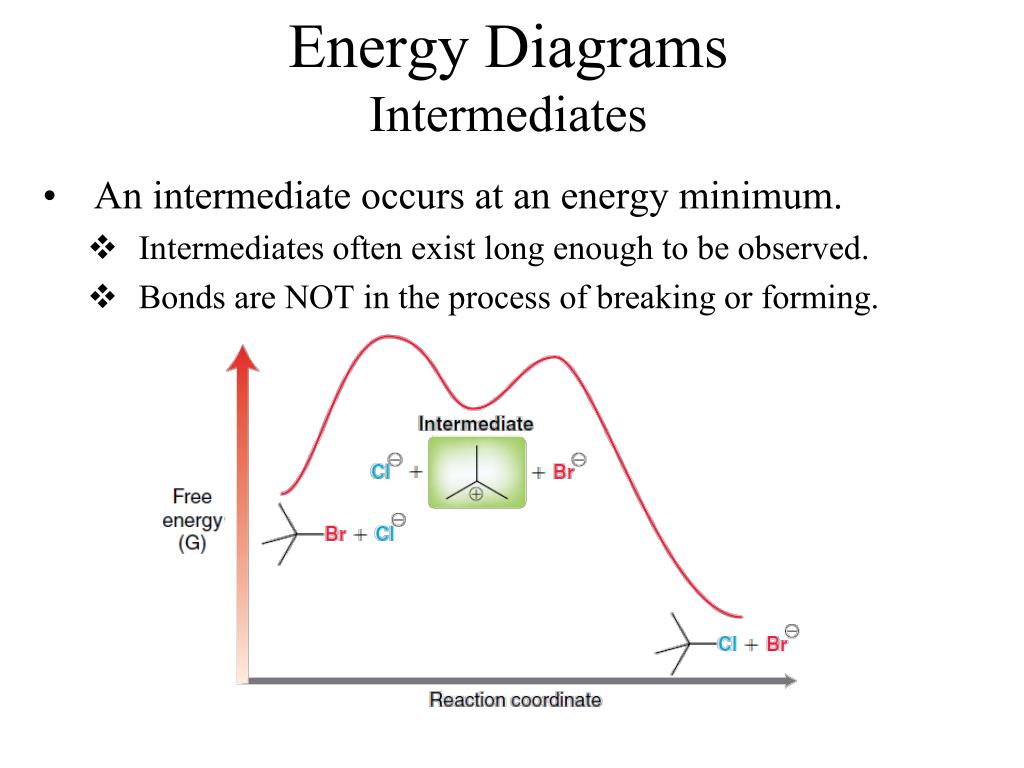
Below is a reaction coordinate diagram for an endothermic reaction. Figure %: Reaction coordinate diagram for an endothermic reaction. If a reaction has n elementary steps in its mechanism, there will be n-1 minima between the products and reactants representing intermediates.
Is there any program/way to nicely and quickly build a diagram so I can have a nice way to visualize all the data? I want any ideas before I do it less than optimally in excel. I don't think there are any fancy ways of doing this, as many articles have crappy reaction coordinate diagrams.
There are many wonderful sign vocabulary resources available online and ASLCORE is happy to contribute in a unique way! The suggested signs you see in each ASLCORE branch are developed according to ASL linguistic principles by fluent Deaf ASL signers.
Reaction Coordinate Diagrams - College Chemistry Here is an energy diagram for the reaction: energy A + (kJ/mol) C+D reaction coordinate Use the energy diagram to answer these questions. k/mol What is the heat of reaction? Exothermic O Endothermic O Neither Is the reaction exothermic...

Activation Barrier To Reaction Process Kinetic Molecular Theory In A Reaction There Must Be A Chemical Changes Reactant Bonds Are Broken Product Bonds Ppt Download



















0 Response to "38 endothermic reaction coordinate diagram"
Post a Comment