38 degrees of freedom phase diagram
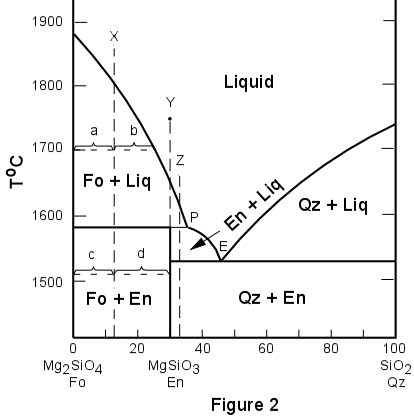
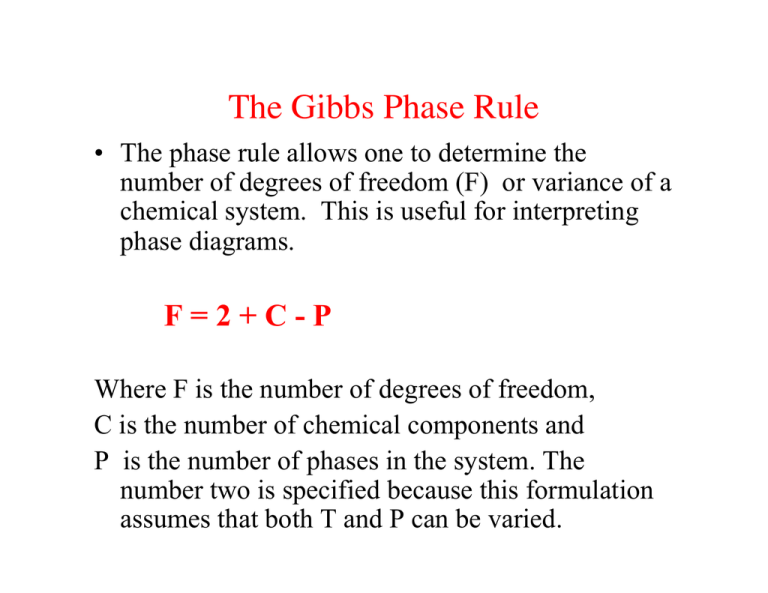
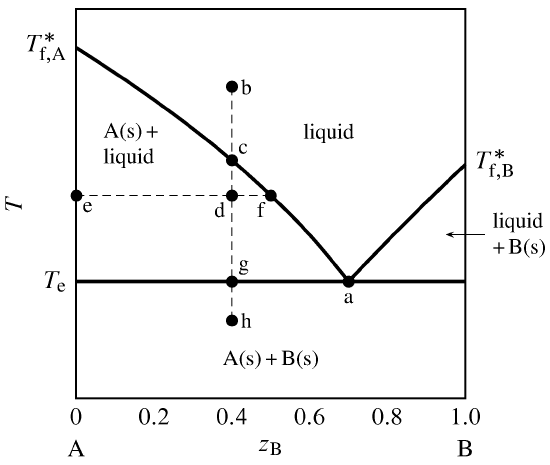
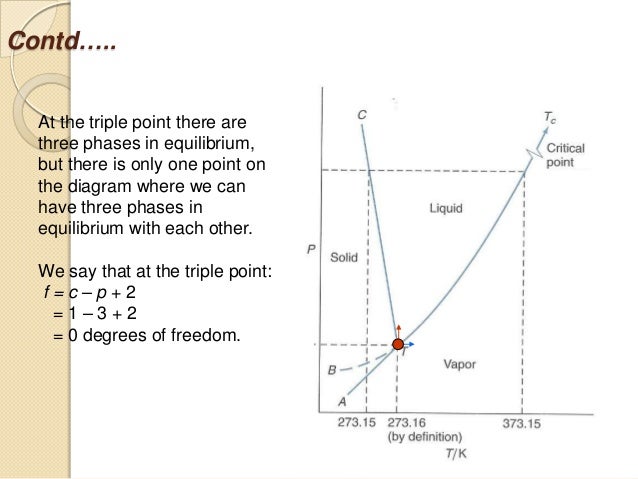
degrees of freedom (F) of the system is related to the number of components (C ) and number of phases (P) by thefollowing phase rule equation : F = C – P + 2 ... The phase diagram for the water system is shown in Fig 6.1. The phase diagram consists of 1.Curves: There are three curves OA, OB and OC. According to Gibbs Phase Rule, at this phase transition, we know that the number of degrees of freedom is <math>F=\left( C+2-P \right)=2+2-3=1 </math>. There is only one degree of freedom, so if we assume constant pressure (a typical choice), then all three phases can only be in equilibrium at an invariant point--- the eutectic point! This ...
The total number of degrees of freedom equals to the total number of variables minus the total number of equations: F = P(C - 1) + 2 - C(P - 1) = C - P + 2 One-component systems F = 3 - P Only one phase is present: F = 2 - an area on a phase diagram. Two phases in equilibrium: F = 1 - pressure is not freely variable if the
Degrees of freedom phase diagram
The composition of both the phase can, therefore, be expressed in terms of the same chemical individual NH 4Cl. Phase Components Solid = NH4Cl Gaseous = x NH3 + x HCl or x NH4Cl Thus dissociation of ammonium chloride is a one-component system. DEGREES OF FREEDOM The term Degree of Freedom represented by F in the phase Rule equation (F = C - P ... Gibbs Phase Rule: F=C-P. Composition is graphed for a particular, constant Temperature and Pressure condition, so our Degrees of Freedom will be entirely based on ratios of components (i.e. mole fractions). Our ternary diagram has three vertices (S,X,W), and defining any 2 of these defines the last. Hence the phases in the diagram only have 2 ... (called degrees of freedom), c is the number of components, and p is the number of stable phases in the system. Unary Diagrams Invariant Equilibrium. ... system, the phase diagram usually has the general appearance of that shown in Fig. 3. The diagram consists of two single-phase fields separated by a
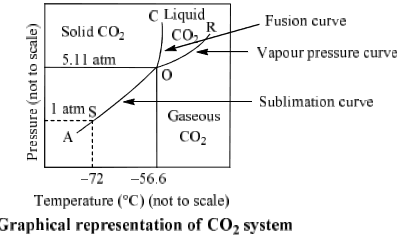
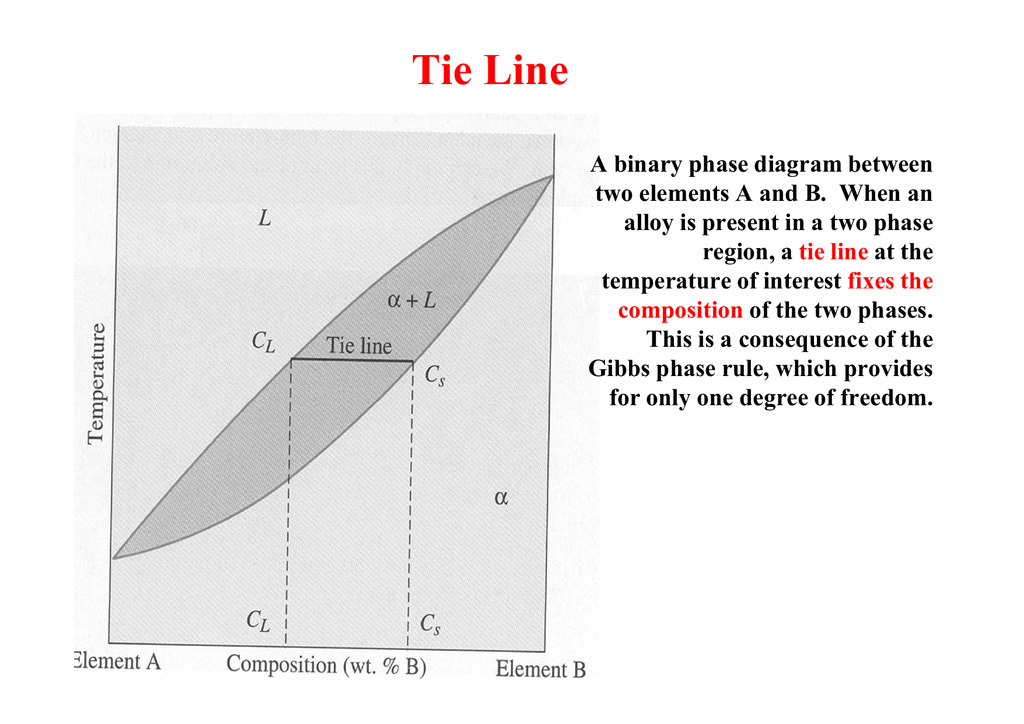
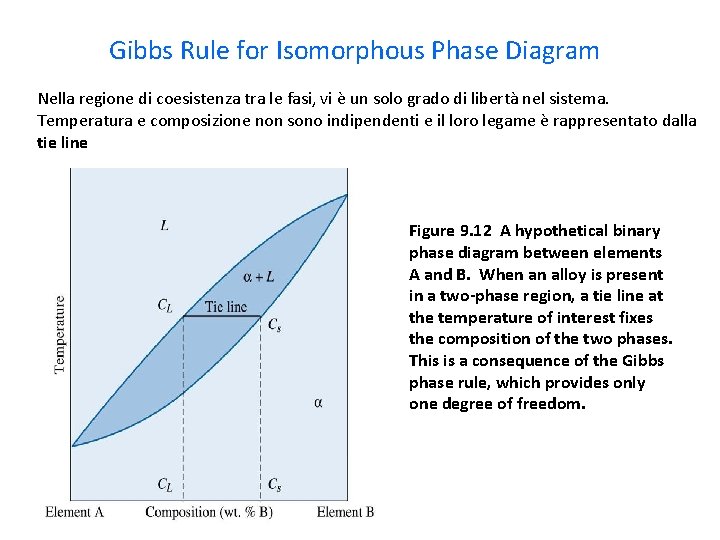
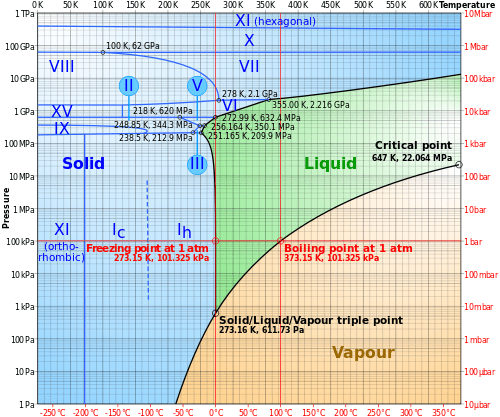
Degrees of freedom phase diagram. In thermodynamics, the phase rule is a general principle governing "pVT" systems (that is, systems whose states are completely described by the variables pressure (p), volume (V) and temperature (T)) in thermodynamic equilibrium.If F is the number of degrees of freedom, C is the number of components and P is the number of phases, then = + It was derived by American physicist Josiah Willard ... According to the phase diagram of pure water its freezing temp is 0 degrees c at 1 atm. The phase rule allows one to determine the number of degrees of freedom f or variance of a chemical system. If the temperature is fixed two degrees of freedom remain. A phase diagram isotherm for a quaternary system with a vapor phase and a liquid phase ... According to the phase rule, a one component system has no degrees of freedom when three phases are in equilibrium (F=0).The system is invariant. The triple point of water is at 273.16 K and 612 Pa. Phase diagram for water showing the triple point of water. 3 single phase regions : Each has three degrees of freedom. Two-phase regions: are between the single phase regions : a +13, Each 2 -phase region has two degrees of freedom. Assume that the temperature of binary AB eutectic reaction > eutectic reaction BC, and so there is a "valley" running from M to
Hence the system has three degrees of freedom. From the definition, a gas or vapour may be completely defined by fixing two variables P and T or V because the third variable may be calculated from the equation PV = RT and will have a certain definite value ( R is a constant), hence the degree of freedom of the system is two. Definitions. Gibbs Phase Rule is expressed by the simple formulation: P + F = C + 2, where. P is the number of phases in the system. A phase is any physically separable material in the system. Every unique mineral is a phase (including polymorphs); igneous melts, liquids (aqueous solutions), and vapor are also considered unique phases. The Gibbs Phase rule can be applied to a P-T thermodynamic diagrams for pure substances. On any such of diagram, each point represents a state of the material. Once the point has been located, all other thermodynamic properties can be read off. For a single phase, the required point can fall anywhere on the region of the plane representing this ... The degrees of freedom of a system dictate the number of phases (as described above in the bullet list) that can occur in the system. Note. The critical point (on a phase diagram) can only exist at one temperature and pressure for a substance or system and thus the degrees of freedom at any critical point is zero.
Gibbs Phase Rule: f = c - p + 2 f = Intensive Degrees of freedom = variance Number of intensive variables that can be changed independently without disturbing the number of phases in equilibrium p = number of phases gas, homogeneous liquid phases, homogeneous solid phases c = components Minimum number of independent constituents Case I. The Gibb's phase rule is find the degree of freedom of the closed system in terms of number of phases in the system and the chemical composition. It is calculated by the given formula:- P+F = C+ 1 2.7: The Gibbs Phase Rule. Print. The Gibbs Phase Rule relates the degrees of freedom in a system to the number of components and number of phases in a system. The Gibbs Phase Rule is: F = C − P + 2. Equation 2.03. Where: F the number of degrees of freedom in the system, integer. C is the number of components in the system, integer. The Gibbs phase rule tells me that at a substance's triple point, where there are 3 phases in equilibrium, there should be 0 degrees of freedom. Based on my understanding, that means there should be 0 intensive properties that can be varied. When you look at a P-T phase diagram, the triple point is actually a point so the 0 DF makes sense.

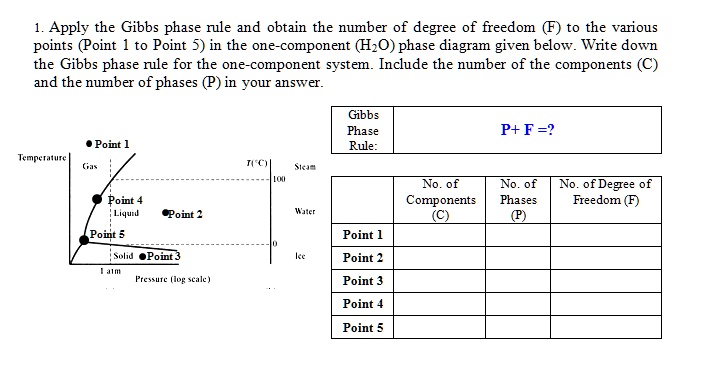
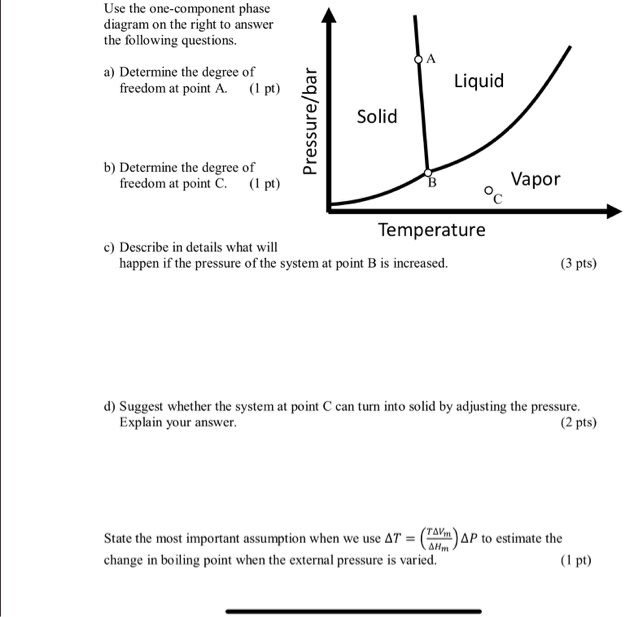
Solved Apply The Gibbs Phase Rule And Obtain The Number Of Degree Of Freedom To The Various Points Oint To Point 5 In The One Component Hzo Phase Diagram Given Below Write Down The
Introduction. In a phase space, every degree of freedom or parameter of the system is represented as an axis of a multidimensional space; a one-dimensional system is called a phase line, while a two-dimensional system is called a phase plane.For every possible state of the system or allowed combination of values of the system's parameters, a point is included in the multidimensional space.
• The phase rule connects the Degrees of Freedom, the number of components in a system and the number of phases present in a system via a simple equation. • To understand the phase rule one must understand the variables in the system along with the degrees of freedom.
1 Answer. The Gibbs phase rule is f=c−p+2, where f is the degrees of freedom, c is the number of components, and p is the number of phases.Your diagram says P=cst, which I interpret to mean that pressure is constant. That specification lowers the number of degrees of freedom by one, so f=c−p+1.
Degrees of freedom phase diagram. F 2 3 1 0 c and t defined only at one point eutectic point no degrees of freedom. If the temperature is fixed two degrees of freedom remain. The critical point on a phase diagram can only exist at one temperature and pressure for a substance or system and thus the degrees of freedom at any critical point is zero.
The goal of the Gibbs phase rule is to describe all the intensive degrees of freedom of a system. It doesn't tell you about extensive degrees of freedom. The extensive degrees of freedom tell you "how much" of the system you have. In this system, the extensive degrees of freedom are the absolute amounts of each phase.
Degrees of freedom phase diagram. Phase diagrams of water co2 explained. A phase diagram isotherm for a quaternary system with a vapor phase and a liquid phase requires therefore an area to mark the concentration range in which a solid phase is in equilibrium with the other two phases. If the temperature is fixed two degrees of freedom remain.
Phase diagram and "degrees of freedom" A phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically-distinct phases; or to show what phases are present in the material system at various T, p, and compositions • "equilibrium" is important: phase diagrams are determined by using slow cooling
Phase Diagrams Best, Chapter 14 Gibbs Phase Rule F = 2 + C - ϕ F = degrees of freedom (P-T-X) C= components ϕ = phases Degrees of Freedom • Rule applies to a phase or assemblage • Divariant indicates two degrees of freedom • Univariant means one degree of freedom • Invariant means there are no degrees of freedom

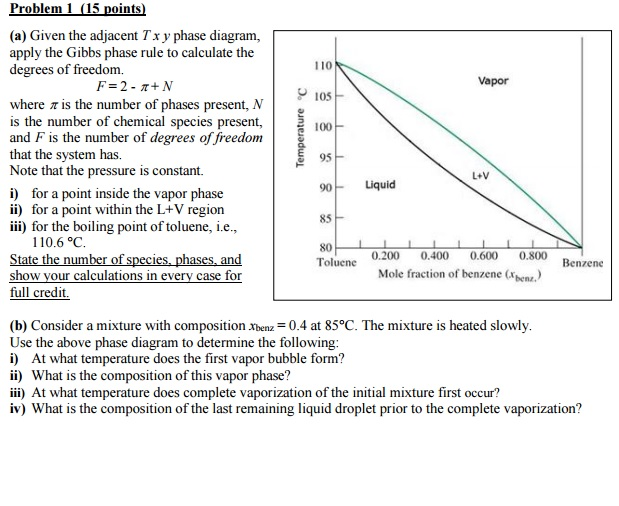
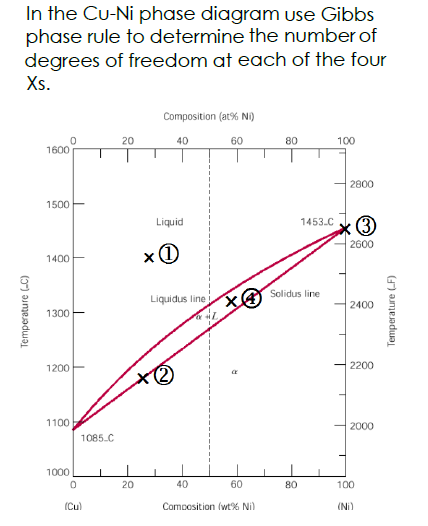
Use Gibbs Phase Rule To Determine The Degrees Of Freedom In A Cu 40 Ni Alloy And Determine The Composition Of Each Phase At The Following Temperatures A 1300 C B 1250 C
as the phase rule: f = c - p + 2, (6.2) where f is the no. of degrees of freedom, p is the number of phases present and the "2" represents the two variables temperature and pressure. Applying this rule to a one-component phase diagram such as that of water, we see that in the interior of the solid, liquid or vapor regions, where only one
5 Jun 2019 — 13.1.1 Degrees of freedom ... Consider a system in an equilibrium state. In this state, the system has one or more phases; each phase contains one ...
The phase rule states that F = C − P + 2. Thus, for a one-component system with one phase, the number of degrees of freedom is two, and any temperature and pressure, within limits, can be attained. With one component and two phases—liquid and vapour, for example—only one degree of freedom exists, and there is one pressure for each ...
Degrees of Freedom. ... This shows qualitatively a very simple phase diagram for a given material. The liquid, solid, and gas phases are separated by curves in the T,P parameter space. On these curves, both phases are represented to some degree simultaneously.
Degrees of freedom phase diagram. Scientists have been able to very accurately account for all the properties of single atoms in sensitive experiments. Understanding degrees of freedom duration. The critical point on a phase diagram can only exist at one temperature and pressure for a substance or system and thus the degrees of freedom at any ...
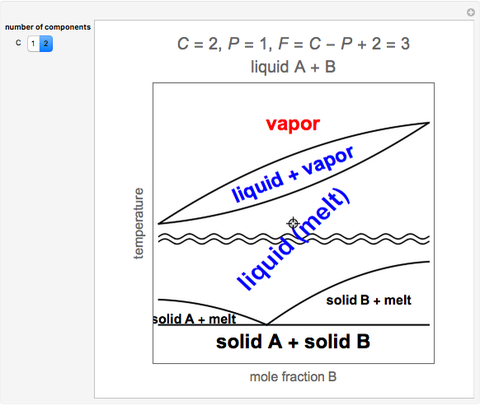
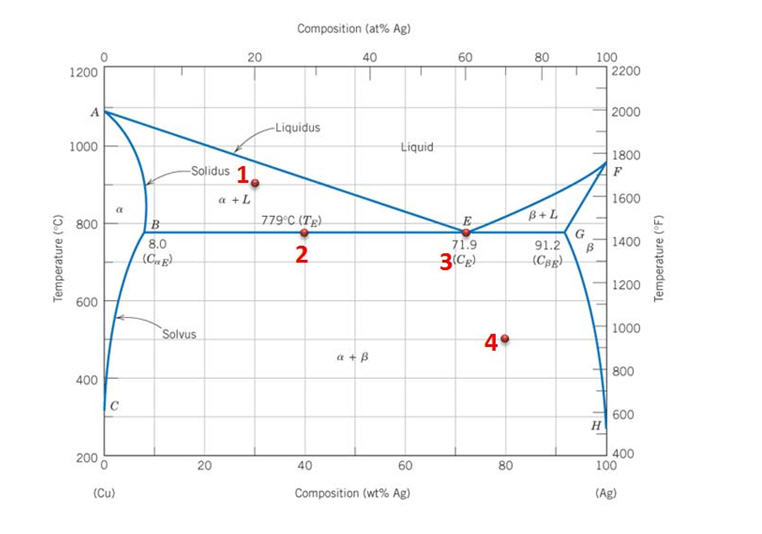
Solved Draw A Phase Diagram Of A Binary System Label All Key Regions And Calculate The Degrees Of Freedom At The Various Regions
The point marking the state of the system in some phase diagram then ... The sum of the number of phases and degrees of freedom thus equals four; P + F = 4.Degrees of freedom F: 2Number of phases P: 1Representation in phase Diagram: Area(s)
(called degrees of freedom), c is the number of components, and p is the number of stable phases in the system. Unary Diagrams Invariant Equilibrium. ... system, the phase diagram usually has the general appearance of that shown in Fig. 3. The diagram consists of two single-phase fields separated by a
Gibbs Phase Rule: F=C-P. Composition is graphed for a particular, constant Temperature and Pressure condition, so our Degrees of Freedom will be entirely based on ratios of components (i.e. mole fractions). Our ternary diagram has three vertices (S,X,W), and defining any 2 of these defines the last. Hence the phases in the diagram only have 2 ...
The composition of both the phase can, therefore, be expressed in terms of the same chemical individual NH 4Cl. Phase Components Solid = NH4Cl Gaseous = x NH3 + x HCl or x NH4Cl Thus dissociation of ammonium chloride is a one-component system. DEGREES OF FREEDOM The term Degree of Freedom represented by F in the phase Rule equation (F = C - P ...














0 Response to "38 degrees of freedom phase diagram"
Post a Comment