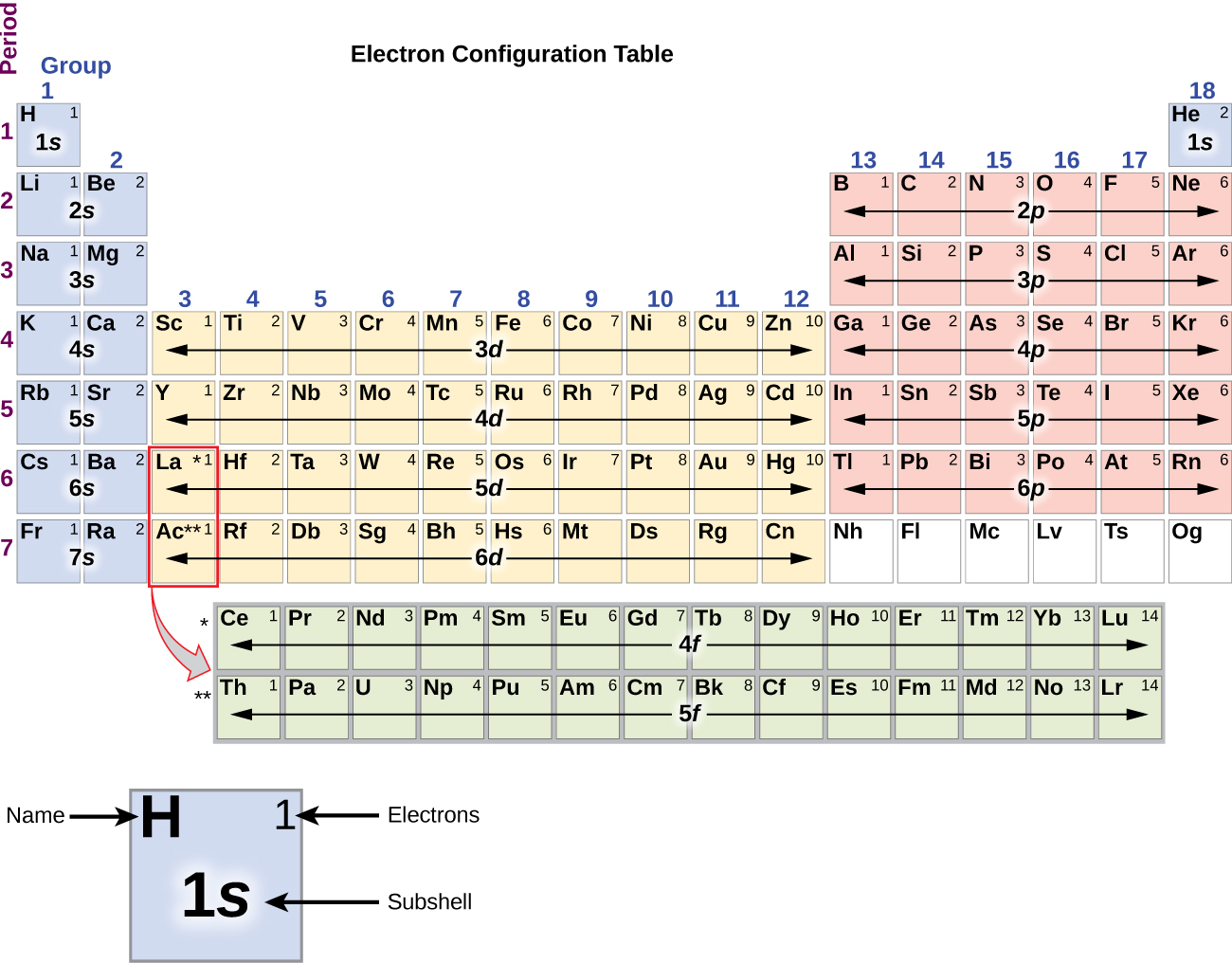
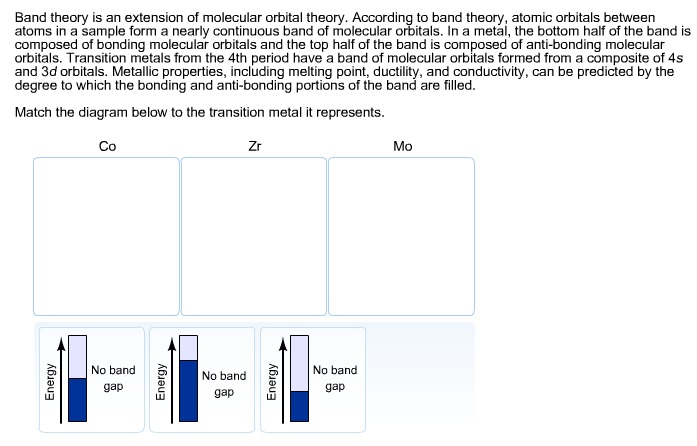
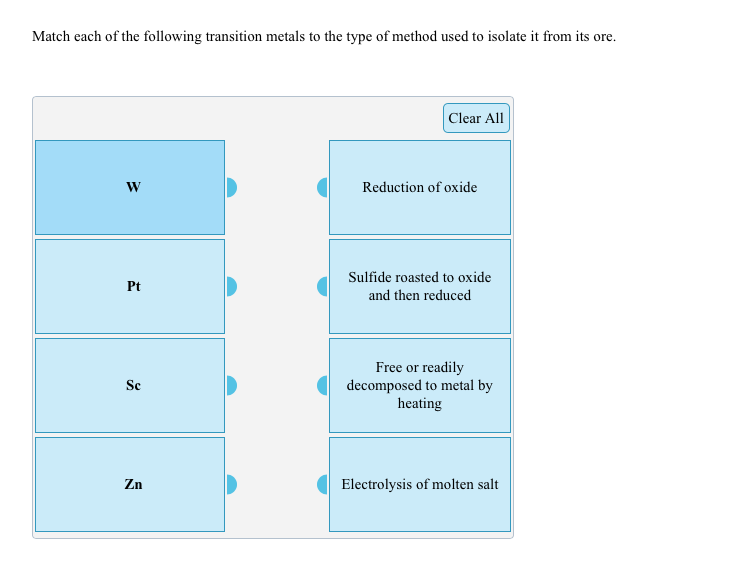
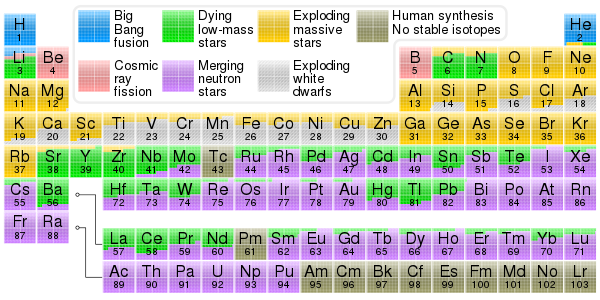
37 match the diagram below to the transition metal it represents.
This photo about: Match the Diagram Below to the Transition Metal It Represents., entitled as Electronic Spectroscopy Interpretation Chemistry Libretexts Match The Diagram Below To The Transition Metal It Represents. - also describes Electronic Spectroscopy Interpretation Chemistry LibreTexts and labeled as: ], with resolution 2758px x 1012px Mar 31, 2016 · Band theory is an extension of molecular orbital theory according to band theory atomic orbital. Which element in figure 5 2 is a transition metal. The Earth Differentiation And Plate Tectonics The thick horizontal lines represent atomic orbitals of the metal left and ligands right. Match the diagram below to the transition metal it represents. These two lets call them sub groups are separated in the periodic table by a special group of highly colorful compounds known as the transition metals.
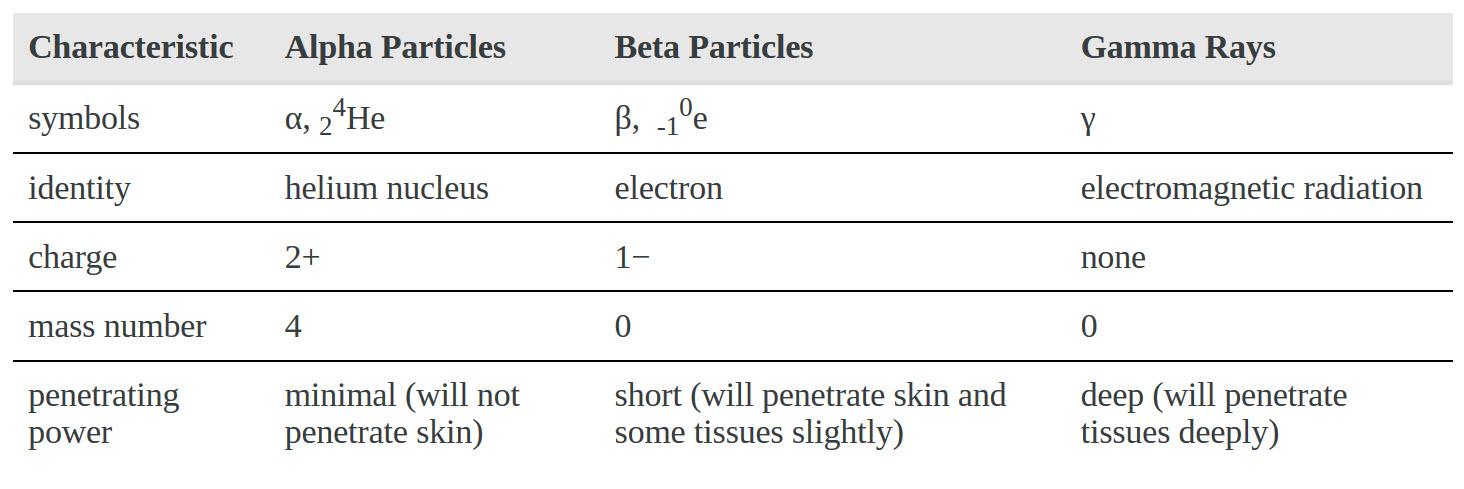
Z represents O2 binding in cells with elevated 2,3-BPG, whereas X represents O2 binding in cells with decreased 2,3-BPG In one type of hemoglobin mutant the amino acid change generates a strong ionic interaction stabilizing the T state conformation but only under conditions of lower pH, e.g., at pH 7.2 compared to pH 7.6.
Match the diagram below to the transition metal it represents.
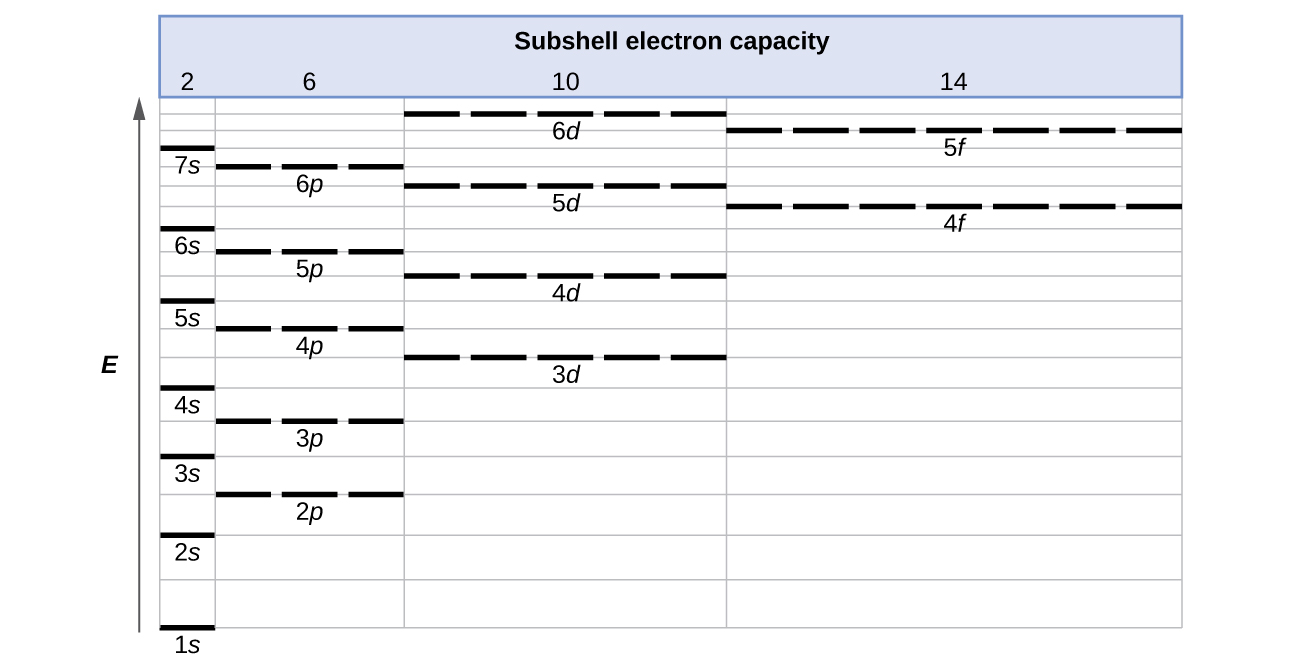
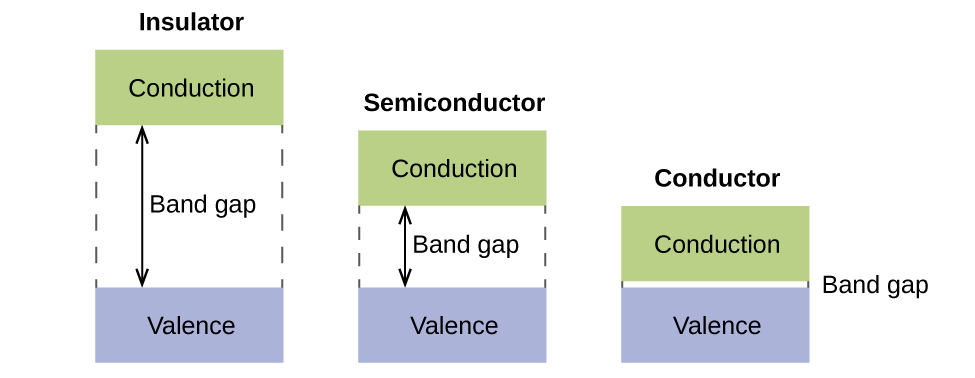
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram . For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. Chapter 9 - 10 Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram 159 ENERGY DIAGRAM - We can map out electrons around an atom using an energy diagram: E N E R G Y 1s 2s 2p 3s 3p 3d 4s 4p 4d 5s 5p Each blank represents an ORIBITAL which can hold up to TWO electrons "1s" means first shell, "s" subshell
Match the diagram below to the transition metal it represents.. Match the Diagram Below to the Transition Metal It Represents. band theory match the diagram to the transition metal it match the diagram to the transition metal it represents what band represents the truest form of metal music which albums by metal mastering chemistry chapter 2 assignment flashcards match each diagram to the atom or ion it represents drag each item to the appropriate bin A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Start studying Chem Exam #2 (Chem 101 Ch 3 hw). Learn vocabulary, terms, and more with flashcards, games, and other study tools. The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. ... The transition state is the point of highest energy between the reactant(s) and product(s). ... such as the platinum metal added to the H 2 /O 2 mixture ...
Sep 29, 2018 · These two lets call them sub groups are separated in the periodic table by a special group of highly colorful compounds known as the transition metals. Match the diagram below to the transition metal it represents. This diagram illustrates the band structure in a 3 rd row metal such as na or mg and how it arises from mo splitting in very small units m 2 m 6. FORMULAS AND NOMENCLATURE OF IONIC AND COVALENT COMPOUNDS Adapted from McMurry/Fay, section 2.10, p. 56 -63 and the 1411 Lab Manual, p. 27 -31. TYPES OF COMPOUNDS Ionic compounds are compounds composed of ions, charged particles that form when an atom (or group of atoms) gains or loses electrons. Jun 25, 2016 · Band theory match the diagram to the transition metal it represents. The diagram below represents information that can be found on the periodic table for the element helium. The transition metals represent groups 1b through 8b of the diagram below and are formed as electrons are filling in the d orbitals. With resolution 2758px x 1012px. According to the Bohr model, the wavelength of the light emitted by a hydrogen atom when the electron falls from a high energy (n = 4) orbit into a lower energy (n = 2) orbit.Substituting the appropriate values of R H, n 1, and n 2 into the equation shown above gives the following result.. Solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental ...
The figure below shows the first ionization energies for elements in the second row of the periodic table. Although there is a general trend toward an increase in the first ionization energy as we go from left to right across this row, there are two minor inversions in this pattern. The first ionization energy of boron is smaller than beryllium ... A structural diagram of what this molecule would look like is shown below. Note that each straight line is being used here to indicate a covalent bond within the phosphate ion. Each straight line represents two electrons (or an electron pair) that is being shared between the atoms. We see from the energy level diagram that the energy levels get closer together as #n# increases. This, the smallest energy and the longest wavelength is associated with the #n = 7 → n = 8# transition. I tried to mark it with an arrow in the diagram, but the lines are so close together that all you can see is the red triangle of the arrowhead. The Lewis acid in coordination complexes, often called a central metal ion (or atom), is often a transition metal or inner transition metal, although main group elements can also form coordination compounds. The Lewis base donors, called ligands, can be a wide variety of chemicals—atoms, molecules, or ions.
• suggested in 1893 that metal ions have primary and secondary valences. ! Primary valence equals the metal's oxidation number ! Secondary valence is the number of atoms directly bonded to the metal (coordination number) Co(III) oxidation state Coordination # is 6 Cl-
The diagram shown below is that for a medium-carbon structural steel. Metallic engineering materials are classified as either ductile or brittle materials. A ductile material is one having relatively large tensile strains up to the point of rupture like structural steel and aluminum, whereas brittle materials has a relatively small strain up to ...
Suppose that an electron makes a transition from a level n i to a level n f (with n i > n f) In order to conserve energy it will have to emit a photon with energy exactly E γ = ΔE = E ni - E nf Quantum mechanically the energy of a single photon is related to its wavelength as E γ = hc/λ Therefore, the wavelength of the emitted photon is:
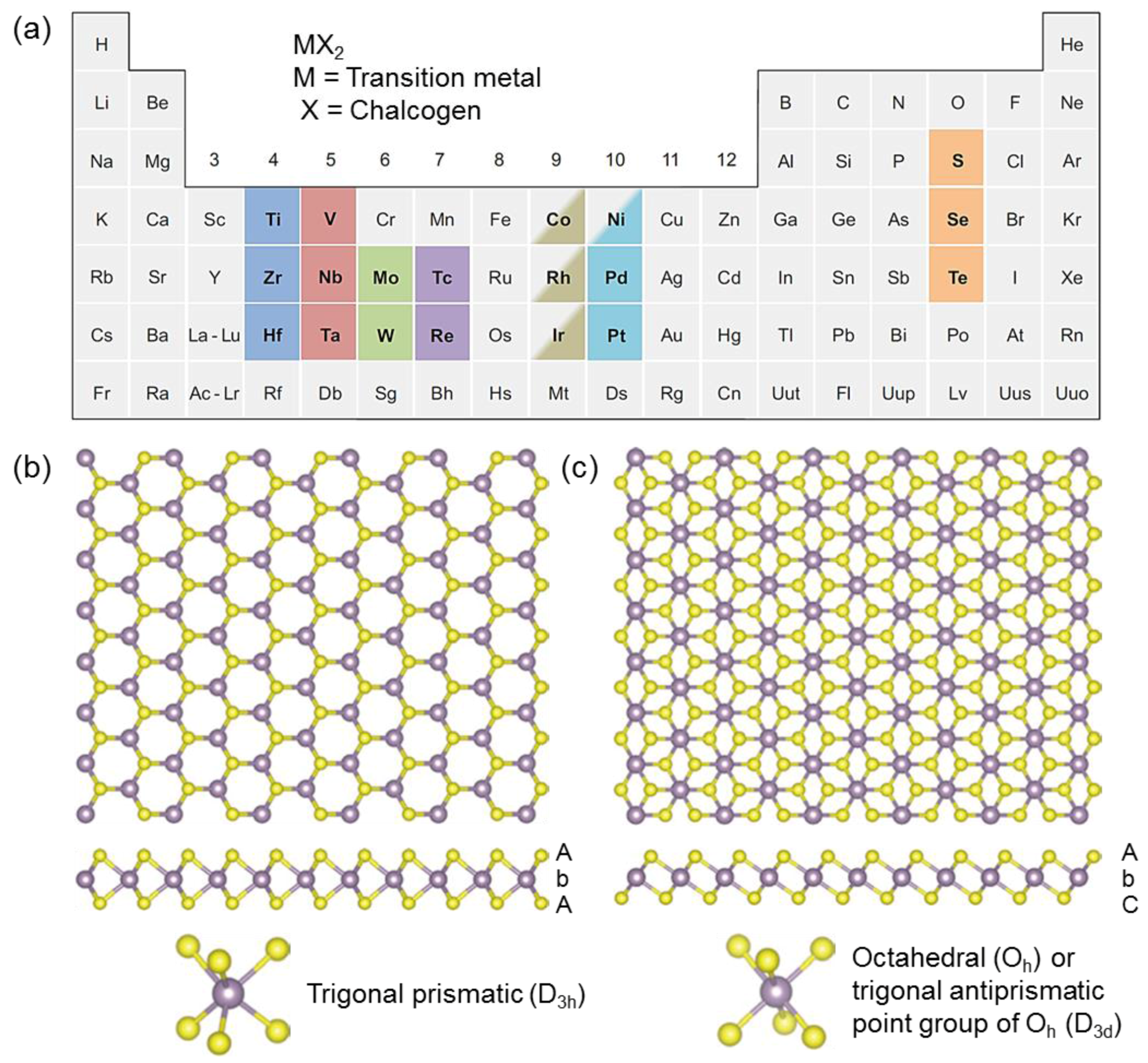
Chemosensors Free Full Text Two Dimensional Transition Metal Disulfides For Chemoresistive Gas Sensing Perspective And Challenges Html
X-rays are produced whenever high-speed electrons collide with a metal target. A source of electrons- hot W filament, a high accelerating voltage between the cathode (W) and the anode and a metal target, Cu, Al, Mo, Mg. The anode is a water-cooled block of Cu containing desired target metal. X-rays glass copper cooling water electrons vacuum
Numerical Analysis On A Viewing Angle Enhancement Of A Digital Hologram By Attaching A Pixelated Random Phase Mask
The _____ sphere is enclosed in brackets in formulas for complex species, and it includes the central metal ion plus the coordinated groups. (a) ligand (b) donor (c) oxidation (d) coordination (e) chelating 2. In coordination chemistry, the donor atom of a ligand is (a) a Lewis acid. (b) the counter ion (c) the central metal atom.
Jan 27, 2017 · Band theory match the diagram to the transition metal it represents. The thick horizontal lines represent atomic orbitals of the metal left and ligands right. Metallic properties including melting point ductility and conductivity can be predicted by the degree to which the binding and anti bonding portions of the band are filled.
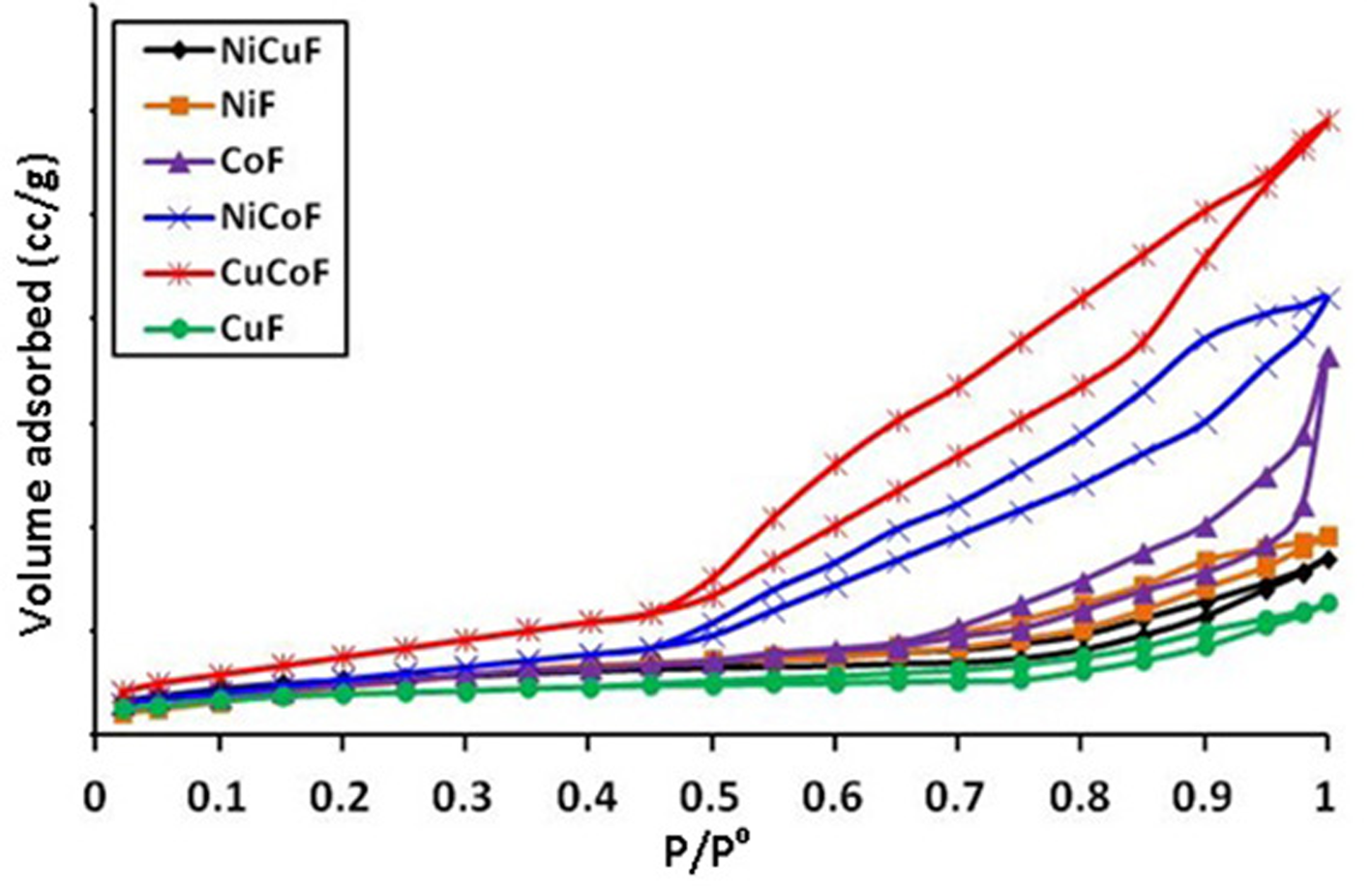
Studies On Characterization Magnetic And Electrochemical Properties Of Nano Size Pure And Mixed Ternary Transition Metal Ferrites Prepared By The Auto Combustion Method Journal Of Materials Research Cambridge Core
Jun 14, 2017 · Match the diagram below to the transition metal it represents. In fact δt 49 δo for the same metal and ligands. The central transition metal atom or ion is grey the six ligands are red and the orbitals are yellow. A give the equation that relates the energy change δe to the planck constant h and the frequency v of the visible light.

Tunable Electronic Structure Of Two Dimensional Transition Metal Chalcogenides For Optoelectronic Applications
The transition labeled "e". 4. Of the five separate electron transitions that have been labeled with letters in the energy-level diagram, which results in the production (or destruction) of the longest wavelength photon? ANSWER. The transition labeled "c". 5. Given that the "Hα" absorption line identified in the hydrogen spectrum ...
Mastering Chemistry: Chapter 2 Assignment. What is the ratio of hydrogen atoms (H) to oxygen atoms (O) in 2 L of water? Enter the simplest whole number ratio in order of hydrogen to oxygen, respectively. Express your answer as two integers, separated by a comma (e.g., 3,4).

Recent Advances And Prospects Of Layered Transition Metal Oxide Cathodes For Sodium Ion Batteries Sciencedirect
The transition metals represent groups 1b through 8b of the diagram below and are formed as electrons are filling in the d orbitals. High school chemistrytransition elements. It is commonly a metal. Match the diagram below to the transition metal it represents. Match each diagram to the atom or ion it represents photos.
11 and position 12. This average diagram is below: metal d orbitals yz xz xy x2-y2 z2 1.125e e 2.75e 0.5e c. The axial/equatorial preference for the -acceptor ligand depends on the number of metal valence electrons. The table below assumes low-spin configurations, reasonable on the basis of the -acceptor ligand.

Light Emission Properties Of 2d Transition Metal Dichalcogenides Fundamentals And Applications Zheng 2018 Advanced Optical Materials Wiley Online Library
Match the diagram below to the transition metal it represents. This diagram shows the field splitting of a metal with ligands in an octahedral configuration. Metallic properties including melting point ductility and conductivity can be predicted by the degree to which the binding and anti bonding portions of the band are filled.
Metallic properties, including melting point, ductility, and conductivity, can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents.
Metallic properties, including melting point, ductility, and conductivity, can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents.
159 ENERGY DIAGRAM - We can map out electrons around an atom using an energy diagram: E N E R G Y 1s 2s 2p 3s 3p 3d 4s 4p 4d 5s 5p Each blank represents an ORIBITAL which can hold up to TWO electrons "1s" means first shell, "s" subshell
Chapter 9 - 10 Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram . For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons.

Metal Organic Frameworks Mofs Beyond Crystallinity Amorphous Mofs Mof Liquids And Mof Glasses Journal Of Materials Chemistry A Rsc Publishing

Recent Progress In Cvd Growth Of 2d Transition Metal Dichalcogenides And Related Heterostructures Zhang 2019 Advanced Materials Wiley Online Library










0 Response to "37 match the diagram below to the transition metal it represents."
Post a Comment