36 bf molecular orbital diagram
Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of ch4 is sp3. Drawing the lewis structure for bf 3. A lewis base is defined as a compound that can donate an electron pair to a lewis acid, a compound that can accept an electron pair. What is Lewis structure of bf3? There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.
MCQ Questions Chapter 4 Chemical Bonding and Molecular Structure Class 11 Chemistry. Question. The hybrid state of S in SO3 is similar to that of. Question. A covalent molecule AB3 has pyramidal structure. The number of lone pair and bond pair of electrons in the molecule are respectively. Question.

Bf molecular orbital diagram
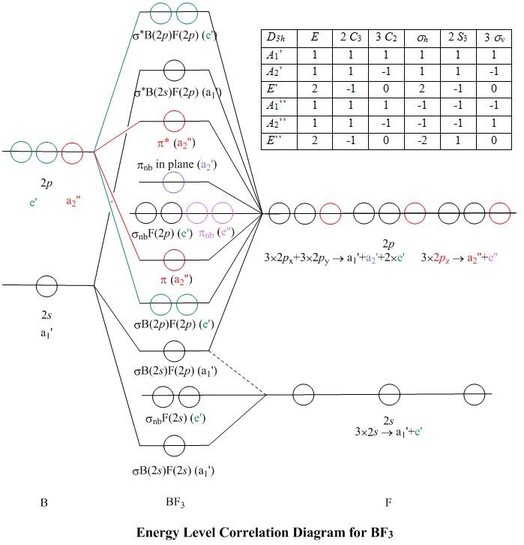
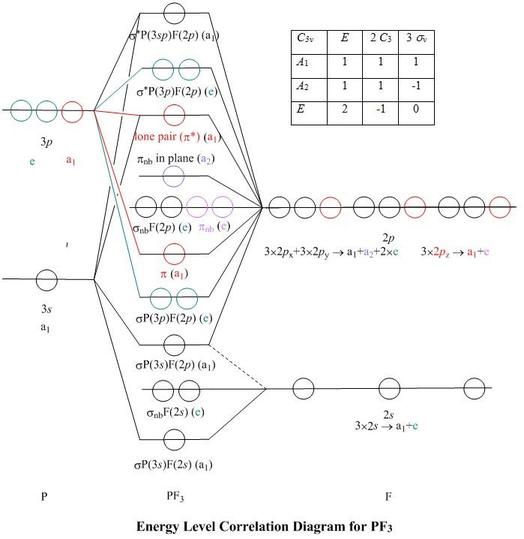
In BF 3, the net dipole moment is zero. Here the resultant of any 2 bond dipoles is equal and opposite to the third. Both ammonia (NH 3) and nitrogen fluoride ... Molecular orbital Diagram. The representation of various M.Os in the increasing order of energy is called M.O diagram. Molecular orbital diagram for BF3. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2p (along the bond axis) for F has an irreducible representation of. The molecular orbital formed by the subtractive effect of the electron waves of the combining atomic orbitals is called an antibonding molecular orbital. Question 50. What is the effect of the process C 2 → C 2 + + e - on bond order of C 2? [A.IS.B. 2002] Answer: Chemical Bonding and Molecular Structure Important Extra Questions Short ...
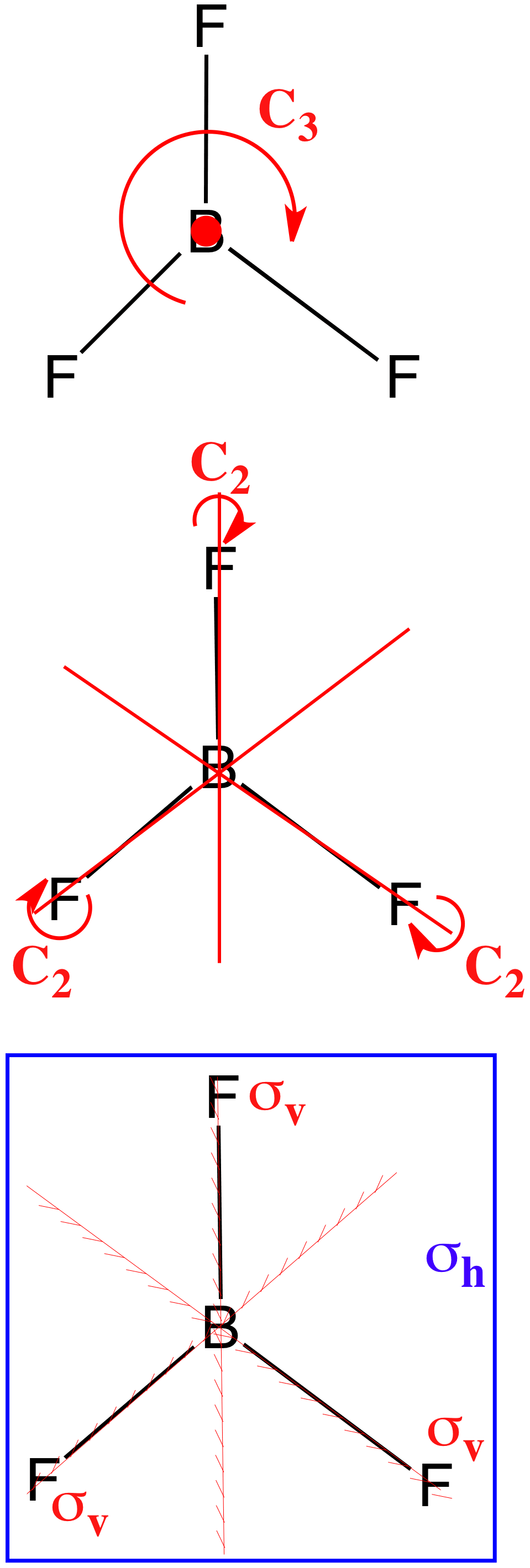
Bf molecular orbital diagram. A complete cleavage of the triple bond of N 2 by fluoroborylene (:BF) was achieved in a low-temperature N 2 matrix by the formation of the four-membered heterocycle FB(μ-N) 2 BF, which lacks a trans-annular N−N bond.Additionally, the linear complex FB=N−N=BF and cyclic FB(η 2-N 2) were formed.These novel species were characterized by their matrix infrared spectra and quantum-chemical ... BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double bond between the Boron and only three σ bonds are formed per Boron atom. The atomic S - orbitals and P - orbitals in Boron outer shell mix to form three equivalent SP2 hybrid orbitals. In BF 3, the net dipole moment is zero. Here the resultant of any 2 bond dipoles is equal and opposite to the third. Both ammonia (NH 3) ... Molecular Orbital Diagram for N 2 Molecular Orbital Diagram for O 2 Bond Order: It is defined as the half of the difference between the number of bonding electrons (N b) and the number ... All the loops (electrons orbitals) are distanced at a 120-degree angle from each other in the same plane. Each orbital gets a single electron in an sp2 loop. The structure formed in the plane suggests that the molecular geometry of BF3 has the shape of trigonal planar (central atoms are surrounded by three-terminal atoms).
BF 3 OEt 2 in toluene at 120 1C for 48 h (Scheme 1 and Fig. S3-S6, ESI†). After purification by column chromatography, BF for-mazanate 8 was isolated as a dark purple solid in 61% yield. Its 1H NMR spectrum lacked the NH resonance previously observed for formazan 7, but showed two doublets at 5.27 and 4.82 ppm due to the diastereotopic CH 2 ... Q.4. Assertion : BF 3 molecule has zero dipole ... pi bonds are formed by the overlapping of p-p orbitals perpendicular to their axis i.e., sidewise overlap. ... The electron density in a bonding molecular orbital is located between the nuclei of the bonded atoms because of which the repulsion between the nuclei is very less while in case of an ... Uncatalyzed and BF 3-catalyzed oxa-Diels-Alder reaction of ethyl vinyl sulfide (EVS) and β-methyl-α-phenylacrolein (ACR) experimentally explored by Ishihara and coworkers is investigated by employing molecular electron density theory.Based on their report, the titled reaction resulted in the formation of two cis and trans cycloadducts with a ratio of 86:14. A series of emissive Cu(I) cationic complexes with 3-(2-pyridyl)-5-phenyl-pyrazole and various phosphines: dppbz (1), Xantphos (2), DPEPhos (3), PPh3 (4), and BINAP (5) were designed and characterized. Complexes obtained exhibit bright yellow-green emission (ca. 520-650 nm) in the solid state with a wide range of QYs (1-78%) and lifetimes (19-119 µs) at 298 K. The photoluminescence ...
BF3, also known as Boron Trifluoride, is an inorganic chemical compound which is a colorless gas with a pungent smell. Students often get confused regarding the polarity or non-polarity of BF3 (Boron Trifluoride) due to the presence of three Fluorine atoms which have a very high electronegativity value when compared to the Boron atom. Chemists explaining a molecule's stability and reactivity often refer to the concepts of delocalization, resonance, and aromaticity. Resonance is commonly discussed within valence bond theory as ... In the molecular orbital diagram for O 2 + ion, the highest occupied orbital is (a) σ MO orbital (b) π MO orbital (c) π* MO orbital (d) σ* MO orbital. Answer. C. Question. The theory capable of explaining paramagnetic behaviour of oxygen is ... BF 3 < NF 3 < PF 3 < ClF 3 ... The formation of the two molecular orbitals from two is orbitals is shown below. Constructive interaction: The two 1s orbitals are in phase and have the same sign, Destructive interaction: The two orbitals are out phase. Question 38. Discuss the formation of N 2 molecule using MO theory. Answer: Molecular orbital diagram of nitrogen molecule (N 2)
Let us discuss how back bonding occurs in BF3 molecules. -BF3 molecule has 2p orbitals of each fluorine which has fully filled orbitals and one of the 2p orbital of boron atoms is vacant. Back bonding BF3 does not affect the bond angle, planarity and the geometry of the molecule. So, the correct answer is "Option C".
MCQ Questions Class 11 Chemistry Chemical Bonding and Molecular Structure with Answers. Question 6 : Main axis of a diatomic molecule is Z. Atomic orbitals p x and p y overlap to form which of the following orbital? (a) π-molecular orbital. (b) σ-molecular orbital.
MCQ of Chemical Bonding and Molecular Structure, Chapter 4, Chemistry, Class 11. Question 1: In a covalent bond formation, Transfer of electrons takes place. Equal sharing of electrons between two atoms takes place. Electrons are shared by one atom only. Electrons are donated by one atom in shared by both atoms.
MCQ Questions Class 11 Chemistry Chemical Bonding and Molecular Structure. Question. Which one of the following is a correct set? (a) H 2 O, sp 3, angular. (b) BCI 3 ,sp 3, angular. (c) NH +4, dsp 2, square planar.
Thus in case of CH4, one 2s orbital and three 2p orbitals of carbon combine to form four hybridized orbitals directed towards four corners of a tetrahedron. It satisfied the observed bond angles in CH4. Therefore, VBT and hybridisation are not different but hybridisation is a part of VBT.
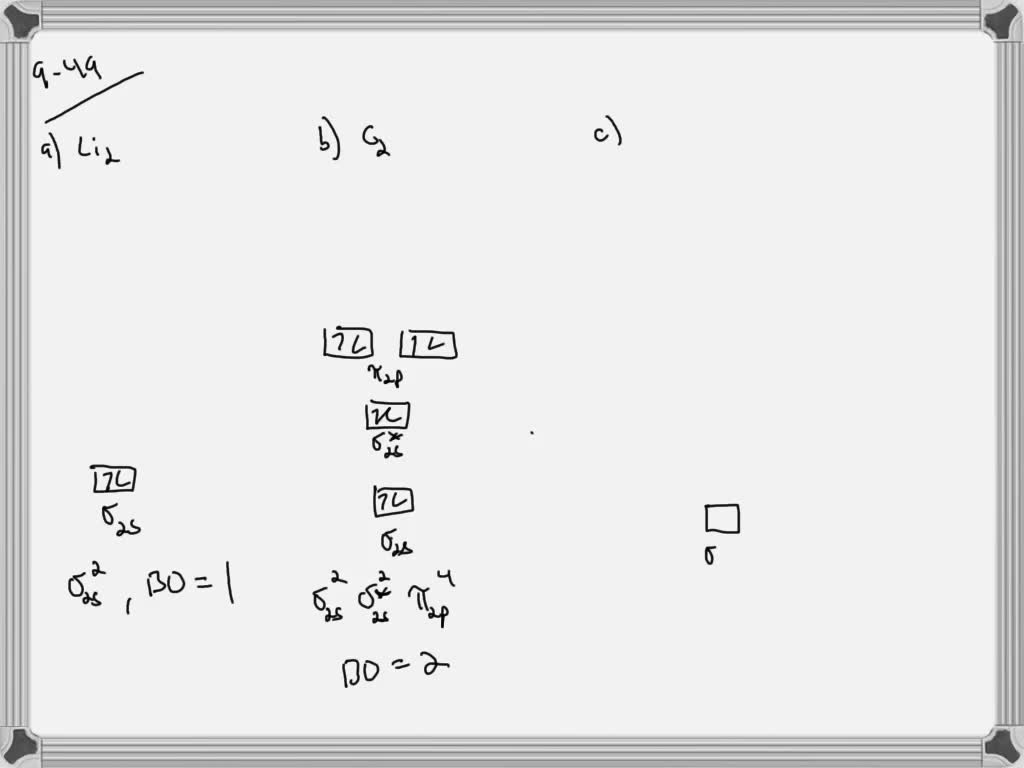
Solved Draw The Molecular Orbital Diagrams For B2 C2 And N2 Please Use It To Predict The Bond Order For B2 B2 B2 C2 C2 C2 N2 N2 And N2 Which
(ii) [BF 4 - and NH 4 +] (iii ... Molecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital (BMO) and anti bonding molecular orbital (ABMO). Energy of anti bonding orbital is raised above the parent atomic orbitals that have combined and the ...
Hope the information shed above regarding NCERT MCQ Questions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure with Answers Pdf free download has been useful to an extent. If you have any other queries of CBSE Class 11 Chemistry Chemical Bonding and Molecular Structure MCQs Multiple Choice Questions with Answers, feel ...
Hope the information shed above regarding NCERT MCQ Questions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure with Answers Pdf free download has been useful to an extent. If you have any other queries of CBSE Class 11 Chemistry Chemical Bonding and Molecular Structure MCQs Multiple Choice Questions with Answers, feel ...
The bond dissociation energy of B—F in BF 3 is 646 kJ mol −1, ... All the molecular orbitals in the dioxygen will be completely filled (c) Total number of bonding molecular orbitals will not be same as total number of anti-bonding orbitals n dioxygen (d) Number of filled bonding orbitals will be same as number of filled anti-bonding ...
a, Transmission spectrum around PA4, for a balanced Fermi gas composed of 8.0 (2) × 10 5 atoms at 832 G with a probe polarization tilted by θ = 26 (inset). The two resonances located around Δ a ...
Tagged as: A molecular orbital with higher energy than the atomic orbitals, A tetra atomic molecule with zero dipole moment is, Bond dissociation energy of a covalent bond decreases with increase, bond length, bond order, chemical bonding and molecular structure mcqs for neet, chemical bonding and shapes of molecules, chemistry, class 11 ...
The molecular orbital formed by the subtractive effect of the electron waves of the combining atomic orbitals is called an antibonding molecular orbital. Question 50. What is the effect of the process C 2 → C 2 + + e - on bond order of C 2? [A.IS.B. 2002] Answer: Chemical Bonding and Molecular Structure Important Extra Questions Short ...
Molecular orbital diagram for BF3. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2p (along the bond axis) for F has an irreducible representation of.
In BF 3, the net dipole moment is zero. Here the resultant of any 2 bond dipoles is equal and opposite to the third. Both ammonia (NH 3) and nitrogen fluoride ... Molecular orbital Diagram. The representation of various M.Os in the increasing order of energy is called M.O diagram.
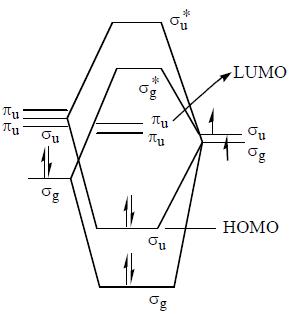
Homo And Lumo In Beh2 Molecules Respectively Area Both Geradeb Both Ungeradec Ungerade And Geraded Gerade And Ungeradecorrect Answer Is Option B Can You Explain This Answer Edurev Chemistry Question

Homework3 Problem 1 A Prepare The Mo Diagram For The Cyanide Ion Cn Use Sketches To Show Clearly How The Atomic Orbitals Interact To Form Mos B What Course Hero

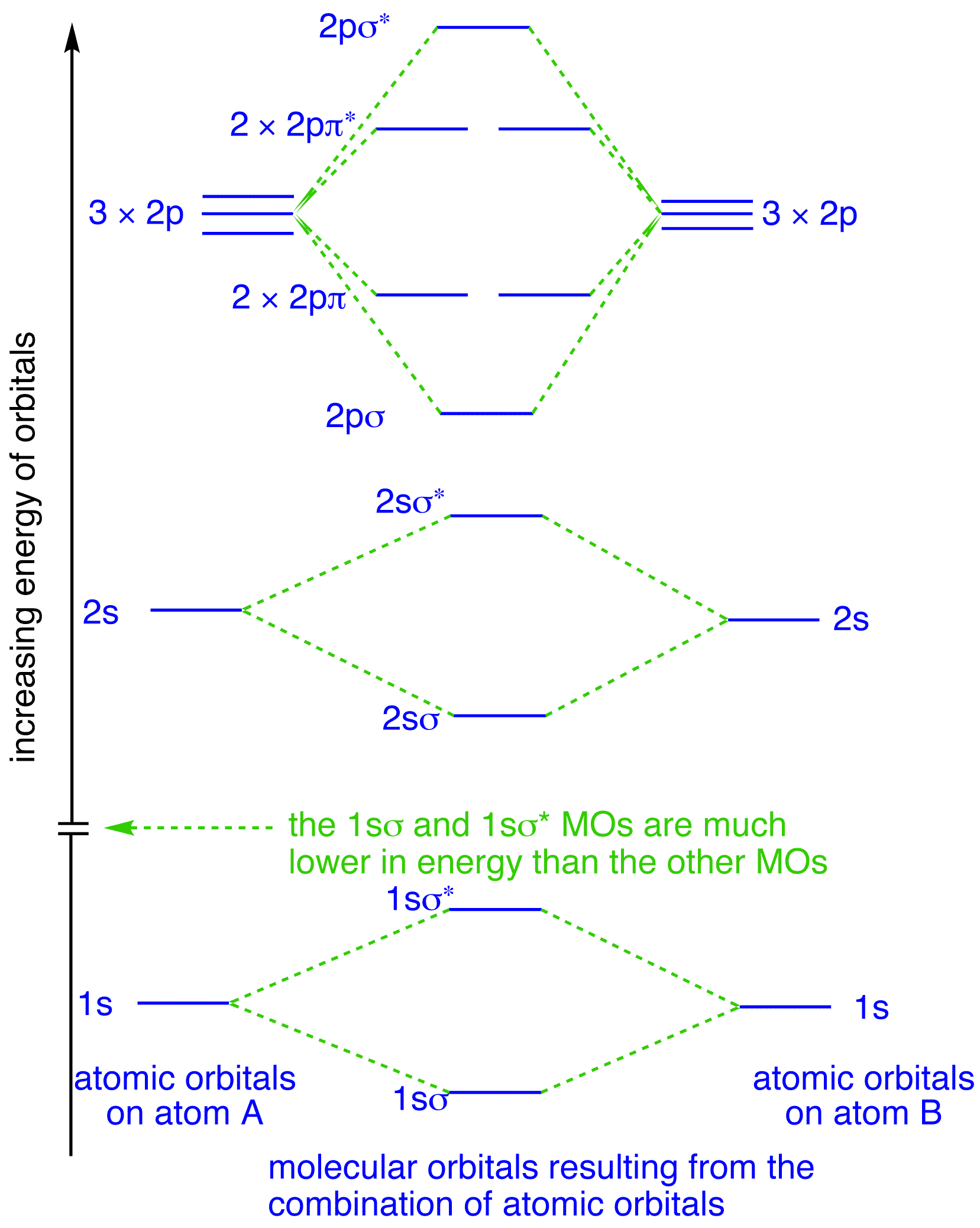


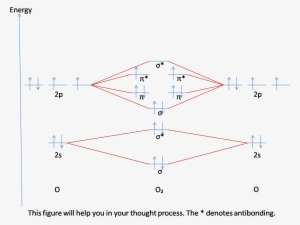





0 Response to "36 bf molecular orbital diagram"
Post a Comment