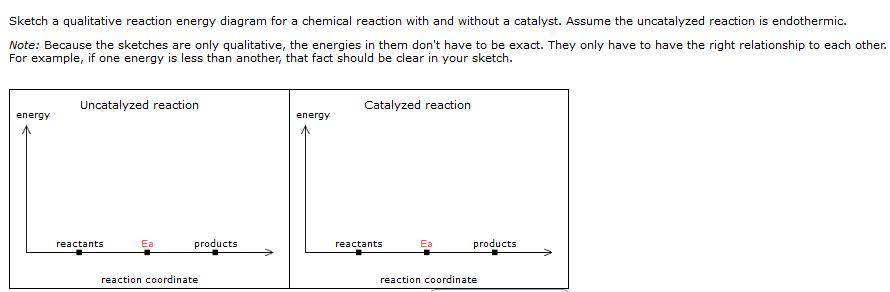
35 reaction coordinate diagram endothermic
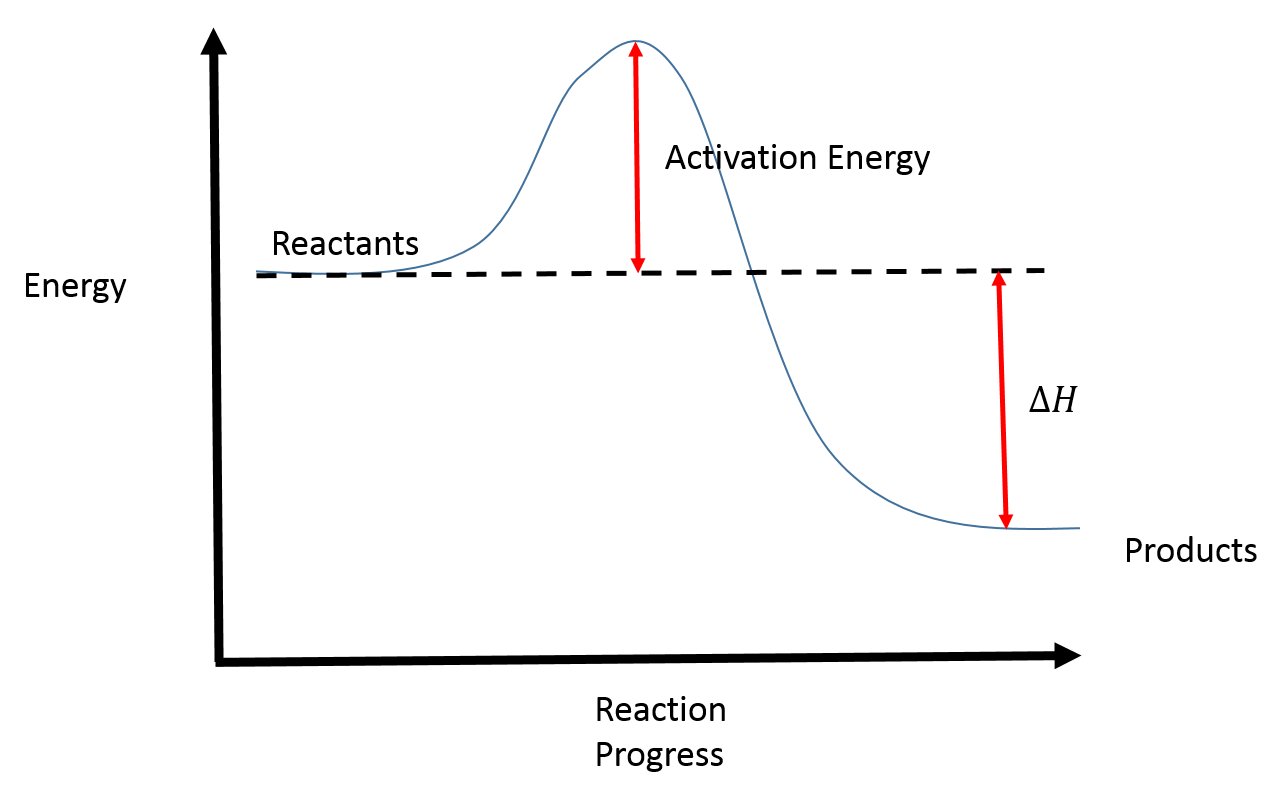
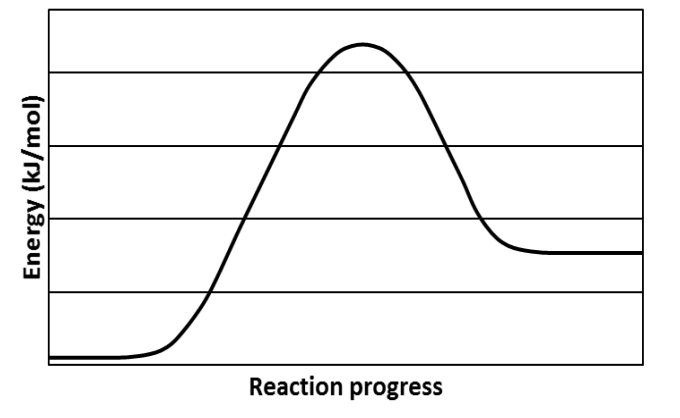
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other. Let's consider a general reaction where a reactant or set of reactants, A, or set of products, B. The diagram below is called a reaction coordinate diagram. B is at a lower total ... Reaction Coordinate Diagram of Ozone Photolysis The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction . Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
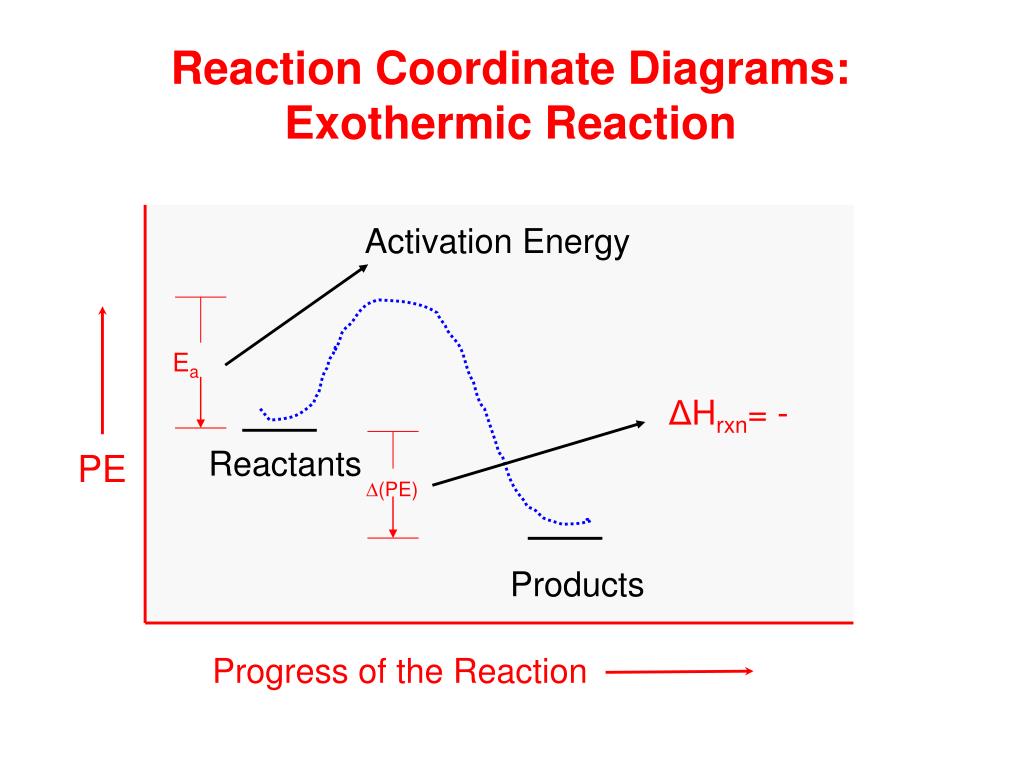
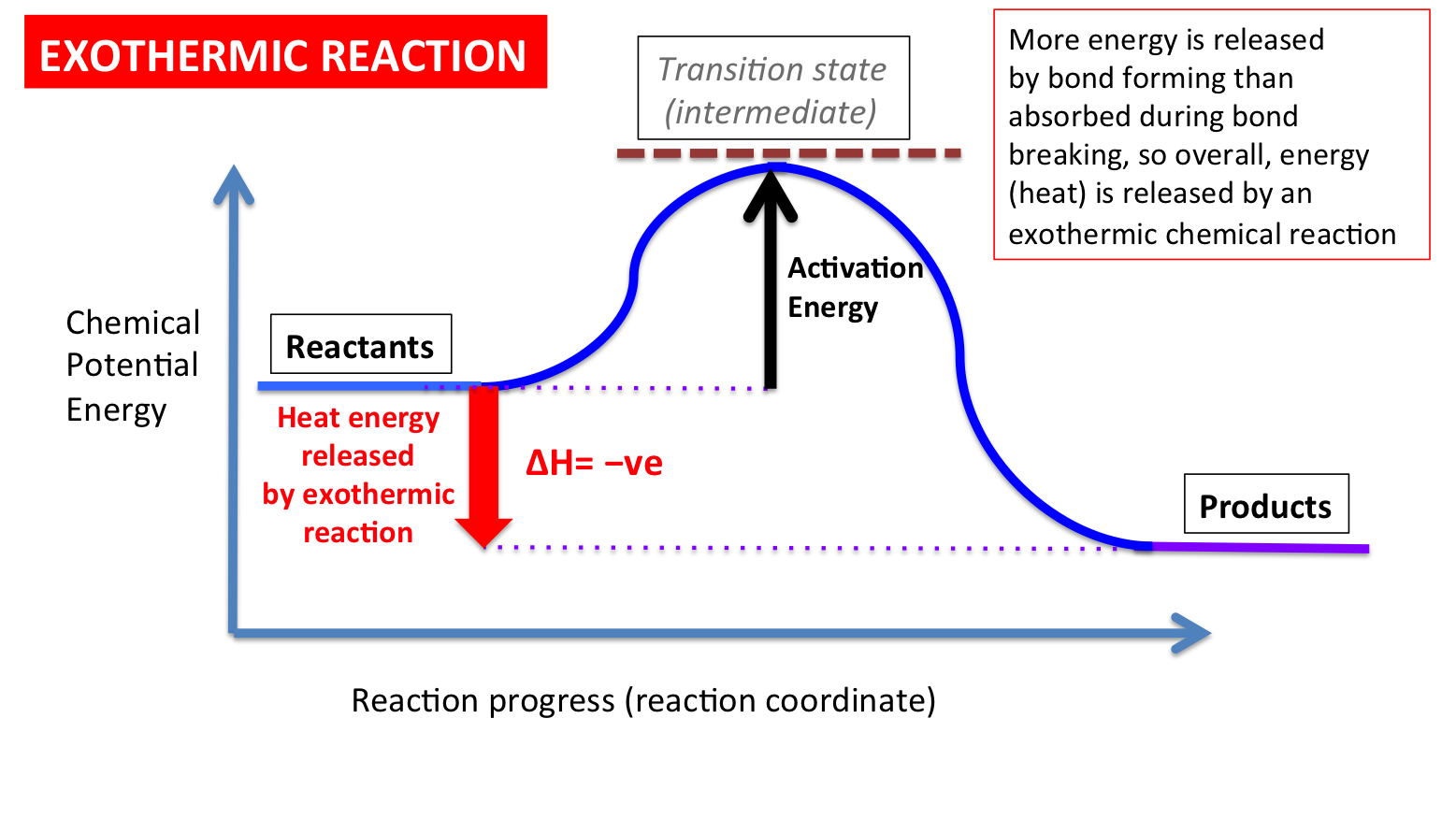
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Reaction coordinate diagram endothermic
Draw a reaction coordinate diagram for a three-step reaction? a 9. Based on the following reaction coordinate diagram, label each reactant (R), transition state (TS), intermediate (1), energy of activation (E.), free; Question: 7. Based on the following reaction coordinate diagram, is the reaction exothermic or endothermic? free energy kJ/mol n ... tables to determine if this reaction is endothermic or exothermic. Construct a reaction coordinate diagram that shows the endothermic or exothermic nature of the reaction and illustrates why this reaction is under kinetic control. Each diagram below (I, Il, Ill, IV) describes a possible reaction: 2 AB(g) A2(g) + B2(g) A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.Start studying ...
Reaction coordinate diagram endothermic. 6. Reaction Coordinate Diagram. Given the following reaction, sketch a reaction coordinate graph. The reaction involves two steps, step 1 is the slowest step and step 2 is the fastest step. Both steps are exothermic. Indicate on the diagram the overall enthalpy change of the reaction, the reaction for the transition states and intermediate states. For endothermic reactions, ones in which energy is absorbed, the potential energy of the products is higher than the ... The diagram below shows the reaction coordinate for a reversible catalyzed and uncatalyzed reaction. Referring to the diagram, answer the questions that follow. 2. 5. 6. 8. 9. The reaction shown above is (a) endothermic, Diagram of a catalytic reaction, showing the energy niveau depending on the reaction coordinate. For a catalysed reaction, the activation energy is lower. In chemistry , a reaction coordinate [1] is an abstract one-dimensional coordinate which represents progress along a reaction pathway. Endothermic reactions: Heat is absorbed. 1) Photosynthesis: Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. 6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2. 2) Cooking an egg: Heat energy is absorbed from the pan to cook the egg.
1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction 5.3. Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of ... Reaction Coordinate Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain why.] According to the diagram, is the po 'al energy of the products greater than, less than, or equal to the potential energy of the reactants? A reaction coordinate diagram is a graph that plots energy versus reaction progress. The amount of energy that needs to be added is called the activation energy, which is the point where the line ...
Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction. Based on the following energy diagram, is the | Chegg.com. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or ... A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions.A reaction will be exothermic if the energy of the products is less than the energy of the reactants. Potential Energy Diagrams Aimver the questions by nferrixig to the diagmm of Aep enezsF of o reaction. 1. ... Reaction Coordinate ... Is the above reaction endothermic or exothermic? r ^ (o^s/C 2. What letter represents the potential energy of the reactants? ^ 3. What letter rejDresents the potential energy of the products?
Oct 01, 2019 · 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic. Figure 8: Reaction Coordinate Diagrams showing favorable or unfavorable and slow or fast reactions [7] The relative stability of reactant and product does not define the feasibility of any reaction all by itself.
Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 .

Plan of Chicago, Chicago, Illinois, Diagram Showing City Growth (1909) // Daniel Hudson Burnham (American, 1846-1912) Edward Herbert Bennett (American, born England, 1874-1954)
406 M-1 min-1 and the rate constant for the reverse reaction is 244 M-1 min-1. The activation energy for the forward reaction is 26.2 kJ mol-1 and that for the reverse reaction is 42.4 kJ mol-1. (a) (5 points) On the axes below, draw a reaction coordinate diagram for this reaction, showing the
General structure of a reaction coordinate diagram, including transition states and intermediates. Overall free energy change and activation energy. Definiti...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Endothermic reaction: heat is consumed by the reaction (+ΔG˚)! Entropy (DS˚): a measure of the freedom of motion! ... (or how does the energy diagram appear)! What we know:! Reaction does not proceed in the dark or in the cold! ... mechanisms have one somewhere along the reaction coordinate! CARBOCATIONS!
Reaction Coordinate Diagram Endothermic. energy profile chemistry for a chemical reaction or process an energy profile or reaction coordinate diagram is a theoretical representation of a single ener ic pathway along energy profiles chemistry tutorial aus e tute energy profiles or energy diagrams for endothermic and exothermic reactions with or without a catalyst tutorial with worked examples for chemistry students.
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea;
Does the following reaction coordinate diagram correspond to an endothermic or exothermic process? С B D Potential Energy A E Reaction Coordinate Endothermic Exothermic ; Question: Does the following reaction coordinate diagram correspond to an endothermic or exothermic process? С B D Potential Energy A E Reaction Coordinate Endothermic ...
Reaction coordinate diagrams. The intrinsic reaction coordinate (IRC), derived from the potential energy surface, is a parametric curve that connects two energy minima in the direction that traverses the minimum energy barrier (or shallowest ascent) passing through one or more saddle point(s). However, in reality if reacting species attains enough energy it may deviate from the IRC to some extent.
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react.
• Energy diagram: A graph showing the changes in energy that occur during a chemical reaction. • Reaction coordinate: A measure in the change in positions of atoms during a reaction. Reaction coordinate Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction.
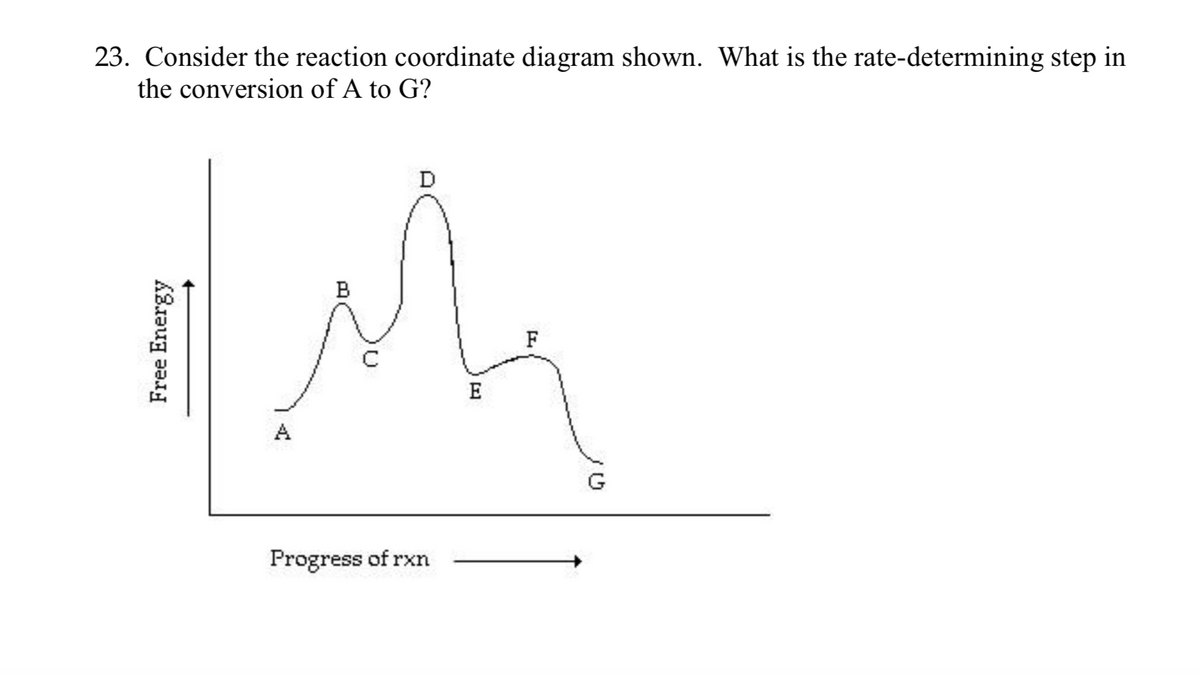
Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat of reaction, locations of transition states, locations of intermediates, and rate-limiting step. 2.
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. In this lesson, we will learn about reaction coordinate diagrams and how they An endothermic reaction is one where at the end of the reaction, energy is put.
A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.Start studying ...
The following reaction coordinate diagram represents left(aright) An;exothermic;reaction left(bright) endothermic;reaction left(cright) Neither;A nor;B left(dright) ∆H;is;less;than;zero | Snapsolve
tables to determine if this reaction is endothermic or exothermic. Construct a reaction coordinate diagram that shows the endothermic or exothermic nature of the reaction and illustrates why this reaction is under kinetic control. Each diagram below (I, Il, Ill, IV) describes a possible reaction: 2 AB(g) A2(g) + B2(g)
Draw a reaction coordinate diagram for a three-step reaction? a 9. Based on the following reaction coordinate diagram, label each reactant (R), transition state (TS), intermediate (1), energy of activation (E.), free; Question: 7. Based on the following reaction coordinate diagram, is the reaction exothermic or endothermic? free energy kJ/mol n ...

860–880 North Lake Shore Drive, 26th Floor and Roof, Plumbing and Ventilation Inst. Riser Diagram (11/22/1949) // Ludwig Mies van der Rohe (American, born Germany, 1886–1969) Associate Architect: Holsman, Holsman, Klekamp and Taylor (American, 20th century) Associate Architect: Pace Associates (American, 20th century) Structural Engineer: Frank J. Kornacker (American, active 1940s–1950s)

Health Sciences Center, Stony Brook, New York, Sectional Diagram (c. 1974) // Bertrand Goldberg American, 1913-1997












0 Response to "35 reaction coordinate diagram endothermic"
Post a Comment