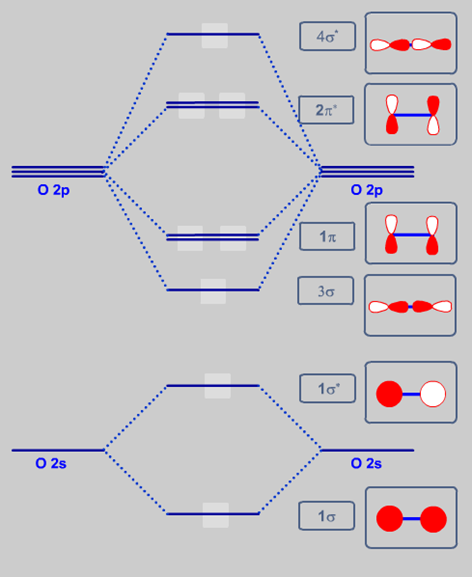
34 use the following mo diagram to find the bond order for o2.
A polyatomic ion is composed of multiple covalently bonded atoms. CO₃²⁻ is a polyatomic ion composed of a carbon atom and three oxygen atoms. Predict the chemical formula for the ionic compound formed by Au³⁺ and HSO₃⁻. Au (HSO3)3. Predict the chemical formula for the ionic compound formed by NH₄⁺ and PO₄³⁻. (NH4)3PO4. Ensure you request for assistant if you can’t find the section. When you are done the system will automatically calculate for you the amount you are expected to pay for your order depending on the details you give such as subject area, number of pages, urgency, and academic level. After filling out the order form, you fill in the sign up ...
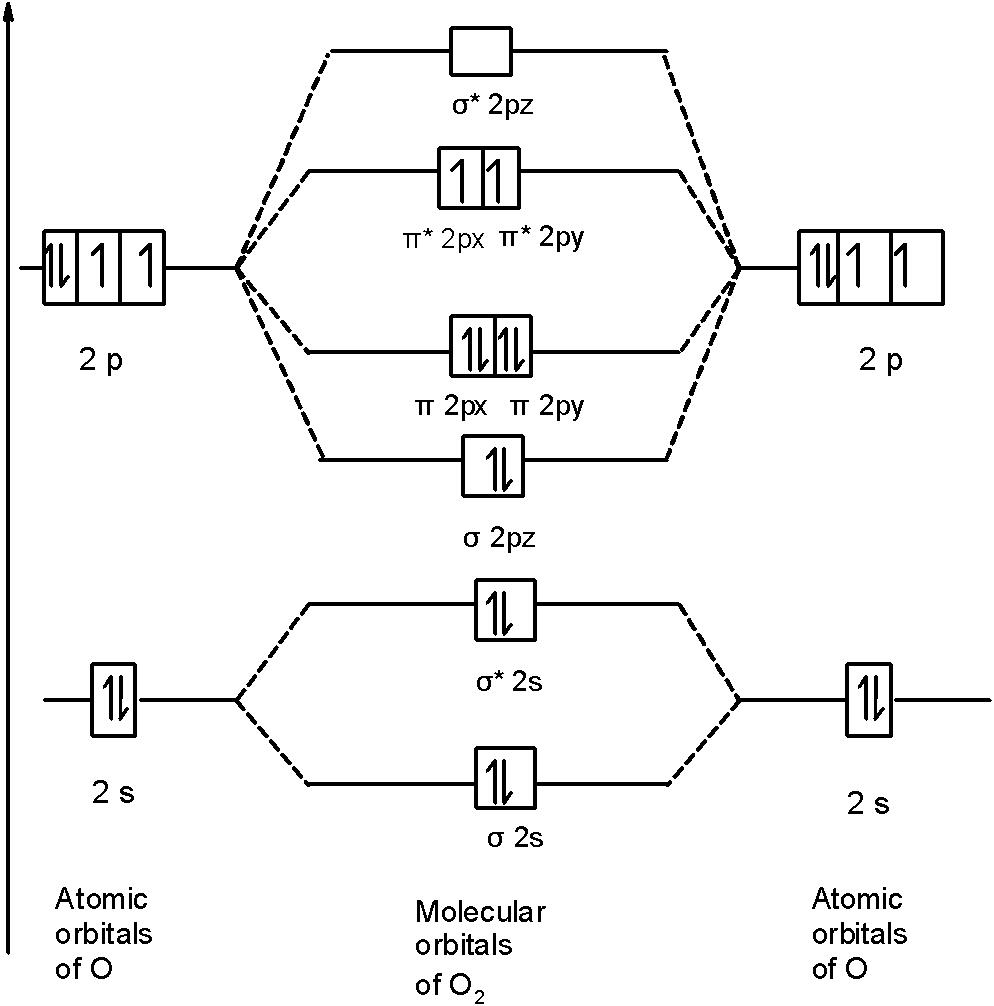
Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O–O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons.
Use the following mo diagram to find the bond order for o2.
The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]` 2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals. Bond Order = ½ ( N b – Na) The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero. Hint: First draw a molecular orbital diagram (MOT) where the atomic orbitals ... To find out the bond order from the molecular orbital configuration is:
Use the following mo diagram to find the bond order for o2.. 7:11For calculation of bond order we use the formula Bond order = no of electrons in bonding - no of electrons ...15 Jun 2020 · Uploaded by Edmerls and the following bond dissociation energies, estimate a value for the C-to-C triple bond dissociation energy. 807 kJ/mol The heat of vaporization of water at 100°C is 40.66 kJ/mol. Calculate the quantity of heat that is absorbed/released when 5.00 g of steam condenses to liquid water at 100°C. The bond order of CO is 3. as. Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication of the stability of a bond. An element ... 2:55It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.1 Jul 2017 · Uploaded by chemistNATE
4:59When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p ...31 Jul 2020 · Uploaded by chemistNATE Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds.Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students. The bond order varies from one molecule to another. Oxygen is a diatomic molecule. Let us first know what is meant by bond order. Bond order. The bond order may be defined as half the difference between the number of electrons in bonding molecular orbitals (Nonbonding) and the number of electrons in the antibonding molecular orbital. Formula ...
No matter what kind of academic paper you need, it is simple and affordable to place your order with Achiever Essays. We have experienced writers in over 70+ disciplines for whom English is a native language and will easily prepare a paper according to your requirements. Order Now Free Inquiry. Calculate your paper price. Type of paper. Academic level. Deadline. Pages (550 words) − ... "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram. M O diagram A has the following orbitals from lowest energy to highest: sigma 2 s, antibonding sigma 2 s, pi 2 p, sigma 2 p, antibonding pi 2 p, and antibonding sigma 2 p. For each of these molecules, identify the proper MO diagram and the number of valence electrons. The 1𝑠 orbital is not shown. Identify the MO diagram for B2. B2 valence e−: Identify the MO diagram for C2. C2 valence e ... 24:30Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook ...26 Oct 2018 · Uploaded by TRICKY CHEMISTRY BASICS BY SUMAN NEGI
4:15Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than ...1 Aug 2020 · Uploaded by chemistNATE
greater bond polarity in BeH2. 5.16 BeF2 uses s and p orbitals on all three atoms, and is isoelectronic with CO2. The energy level diagram for CO2 in Figure ...29 pages
Explanation: In a molecule, there are total 16 electrons. The molecular orbital configuration of molecule is as follows.. The formula for bond order is as follows. Bond order = There are 10 bonding and 6 non-bonding electrons in the orbitals according to the molecular orbital configuration.
We have a convenient order form, which you can complete within minutes and pay for the order via a secure payment system. The support team will view it after the order form and payment is complete and then they will find an academic writer who matches your order description perfectly. Once you submit your instructions, while your order is in progress and even after its completion, our support ...
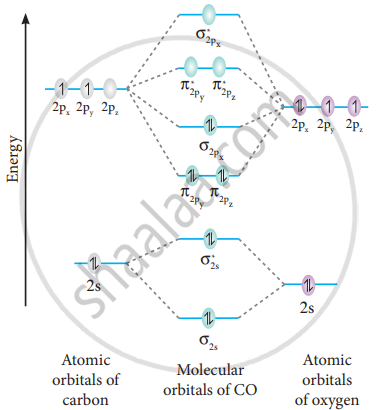
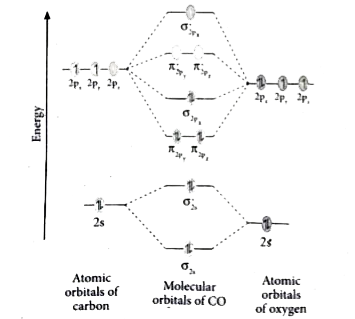
Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2.
The following is the diagram for the neutral oxygen. The bond order is shown for the neutral oxygen. To figure it out for the positive ion simply remove one ...5 answers · 31 votes: Hello! I actually just covered this question in my gen chem class this week. I have attached ...
Without 2s-2p mixing With 2s-2p mixing CK :O r 25 20 2s AO MO AO AO MO AO A MO energy levels for O Fa. and Ne B MO energy levels for Ba Cz and Ng 0.5 This problem has been solved! See the answer See the answer See the answer done loading
Solution. , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O.

Use Mo Diagrams And The Bond Order From Them To Answer Each Of The Following Questions A Is O2 Stable Or Unstable B Is Be2 Diamagnetic Or Paramagnetic Study Com
We then use a plagiarism-detection software to ensure that it is, actually, completely plagiarism free. We ensure that there is no way you could find your paper plagiarized. Calculator. Calculate the price of your paper . Type of paper needed. Pages. − + Academic level. Deadline. Currency. Total price: $26. Continue to order. Free features. Formatting (APA, MLA, Harvard, Chicago/Turabian ...
Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community
Ensure you request for assistant if you can’t find the section. When you are done the system will automatically calculate for you the amount you are expected to pay for your order depending on the details you give such as subject area, number of pages, urgency, and academic level. After filling out the order form, you fill in the sign up ...

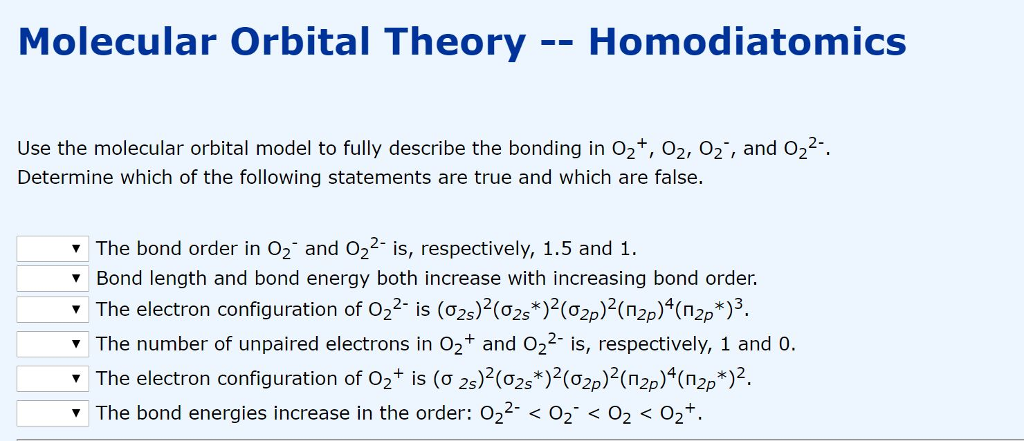
Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are
Hint: First draw a molecular orbital diagram (MOT) where the atomic orbitals ... To find out the bond order from the molecular orbital configuration is:
2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals. Bond Order = ½ ( N b – Na) The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero.
The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
Symmetry Free Full Text Chemical Reasoning Based On An Invariance Property Bond And Lone Pair Pictures In Quantum Structural Formulas Html

Write The Molecular Orbital Diagram Of N2 And Calculate Their Bond Order Chemistry Topperlearning Com Qbqjy
Draw The M O Diagram For Oxygen Molecule And Calculate Its Bond Order And Show That O2 Is Paramagnetic Sarthaks Econnect Largest Online Education Community
Draw Mo Diagram Of Co And Calculate Its Bond Order Sarthaks Econnect Largest Online Education Community
Write The Electronic Configuration Energy Level Diagram For The Molecular Orbitals Of Oxygen Molecule O2 Sarthaks Econnect Largest Online Education Community

















0 Response to "34 use the following mo diagram to find the bond order for o2."
Post a Comment